| |
MECHANISM OF WATER SYNTHESIS UNDER Pt/Pt TRIBOLOGICAL SYSTEM IN VACUUM
C. Kajdas*1, K. Hiratsuka*2 and M. Hashimoto*2
*1 Institute of Chemistry in Plock,
Warsaw University of Technology, POLAND
*2 Department of Mechanical Science and Engineering,
Chiba Institute of Technology, JAPAN
* — Published in : Kajdas, C., Hiratsuka, K. and Hashimoto, M. (2004), "Mechanism of Water Synthesis under Pt/Pt Tribological System in Vacuum." Tribology: Science and Applications, Herman, M. A, ed,, to be published by Polish Academy of Sciences, Warsaw.
Abstract
Synthesis of water from molecular hydrogen and molecular oxygen is catalyzed by platinum. Earlier Hiratsuka et al. clearly demonstrated that the water formation process from H2 and O2 is enhanced by the friction process. However, the existing model of the reaction process does not account for the chemical reaction mechanism. The aim of this research is to account for the detailed reaction mechanism under friction conditions in vacuum.
It is hypothesized that the water synthesis process under sliding conditions is enhanced by low-energy electrons emitted from the tribological contact. This hypothesis is in line with the negative-ion-radical action mechanism (NIRAM), as proposed by Kajdas. To get better understanding of the (i) mechanical action and (ii) heat effect on this catalytic process, experiments have been designed to obtain adequate results for comparison of the effect of mechanically treated platinum catalyst with the thermally treated catalyst.
To carry out the designed experiments, special vacuum chamber containing a pin-on-disk tribometer, was used. The system consists of two sections at different vacuum level. Partial pressure of each gas was determined by the quadrupole mass spectrometer with a 1 s data sampling time. The same vacuum chamber system was applied to observe the water synthesis process without friction. In this case the catalyst was used in the wire form. To initiate the catalytic reaction under static conditions electric heating was applied.
On the basis of the obtained results a new reaction mechanism is proposed. This specific mechanism is based on the fact that most of the hydrogen absorbed in platinum exists in the atomic form. On the other hand, it has been taken into account that under friction conditions low-energy electrons are emitted.
Key-words: catalysis, platinum, triboelectrons tribochemistry, tribocatalysis
Introduction
Chemical reactions initiated by friction process relate to tribochemistry. Tribochemical reactions are distinct from those of thermochemical ones [1]. The same is due to catalysis and tribocatalysis [2]. Most surface catalytic reactions and the formation of surface intermediates involve charge transfer, either an electron transfer or a proton transfer. These processes are often viewed as modified acid-base reactions [3]. The electron transfer capability of a catalyst is expressed according to the Lewis definition. A surface site having a free pair of electrons that can be transferred to the adsorbate is a Lewis base. A site capable of receiving a pair of electrons from the adsorbate is a Lewis acid. Considering now the negative-ion-radical action mechanism (NIRAM) approach [4] and the NIRAM-HSAB (Hard and Soft Acids and Bases) concept [5] it is possible to look for similarities and differences between tribochemistry and catalysis. Referring to [2] one can say that there is a link between tribochemistry and catalysis and/or tribocatalysis. The present work considers platinum catalyzed water synthesis from hydrogen and oxygen under both friction and non-friction conditions.
Synthesis of water is catalyzed by platinum. In this case the catalyst is in different physical state from that of reactants, hydrogen and oxygen. It is a good example of heterogeneous catalysis. Catalysts of heterogeneous processes work by lowering the activation energy of a reaction. Any substance being a catalyst increases the rate of the chemical reaction, but remains chemically unchanged at the end of the reaction. Actually, one of the most important functions of a catalyst is to help in fast achieving chemical equilibrium for certain chemical reactions. The considered water synthesis reaction is one of the simplest catalytic processes, however, very important and according to the present authors it is not well understood when the classic water catalytic synthesis is compared with the friction catalysis. Additionally, it is a well known fact that just by dropping high surface-area platinum gauze into the hydrogen and oxygen mixture, the reaction occurs instantaneously.
Both substrates have large activation energies for several of the elementary steps for the reaction in the gas phase. Dissociation energies are very large compared with thermal energies, 412 kJ/mol for hydrogen and 468 kJ/mol for oxygen. The subsequent atom-molecule reactions for either H• + O2 or H2 + O• still require an activation energy of about 40 kJ/mol [6]. In the presence of a properly structured platinum surface both molecules dissociate to atoms with zero activation energies (2Pt+H2 ® 2Pt-H, or 2Pt+O2 ® 2Pt-O), as demonstrated by low-pressure surface studies [7, 8].
Considering now hydrogen/oxygen and water adsorption on metals under friction conditions it is to emphasize that generally oxygen and water molecules are adsorbed on metal surfaces, and hydrogen molecules are desorbed from metal surfaces during sliding [9]; much more adsorption of oxygen is observed during mild wear than during severe wear. Accordingly, it can be said that the metal surface activated by the adhesive wear has high activity in oxygen chemisorption. Results of earlier papers published in Japanese [10,] demonstrated the enhancement of chemical reactivity with the adhesive wear in such reactions as oxidation of hydrogen, dehydrogenation of ethylene, oxidation of carbon monoxide and oxidation of nitrogen. Hiratsuka et al. [12] clearly showed that the water generation process from H2 and O2 is enhanced by the friction process in which contact solids were made from high purity platinum (99.99%). That enhanced catalysis was called friction catalysis. Figure 1 depicts the rates of oxygen chemisorption and water desorption as a function of oxygen/hydrogen. Interestingly, the maximum water desorption rate was obtained at the oxygen/hydrogen ratio of around 12. This can be accounted for by the fact that Pt is known to absorb easily hydrogen. That fact allows to assume that the major water part is formed from chemisorbed oxygen atoms and hydrogen atoms sitting in the catalyst bulk.
General working hypothesis
The working hypothesis of the present paper is that in both tribochemical reactions and some heterogeneous catalytic reactions the intermediate reactive species are formed by the same mechanism mostly governed by the negative-ion-radical action mechanism (NIRAM) approach. This hypothesis will be tested on the Pt catalyzed water synthesis example by comparison of test results obtained under friction conditions and without friction.
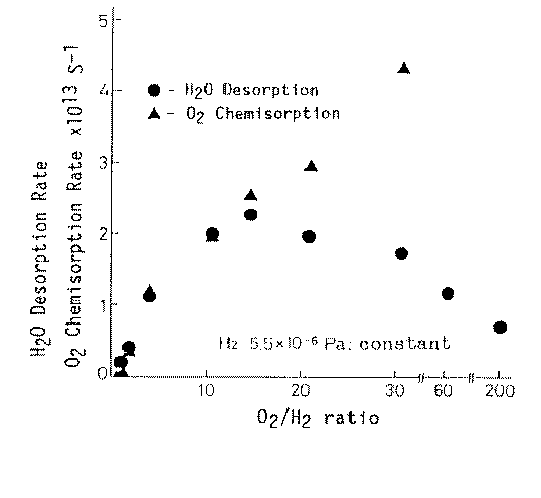 | |
Figure 1. Friction catalysis in the water synthesis during adhesive wear [12] |
Aim of the work and hypothesis
Although the discussed study results [12] clearly evidenced that the Pt catalyzed water synthesis reaction is enhanced by the sliding friction, model of reaction process, as presented in Figure 2, does not account for the chemical reaction mechanism. Thus, the aim of this research is to account for the detailed reaction mechanism of water synthesis from hydrogen and oxygen on platinum catalyst under friction conditions in vacuum.
It is hypothesized that the water synthesis process under sliding conditions is enhanced by low-energy electrons emitted from the tribological contact. This hypothesis is in line with the negative-ion-radical action mechanism (NIRAM) as proposed by Kajdas [2]. To get better understanding of the mechanical action and separately only pure heat action on this catalytic process, experiments have been designed to obtain adequate results for comparison of both the effect of mechanically treated platinum catalyst with the thermally treated catalyst.
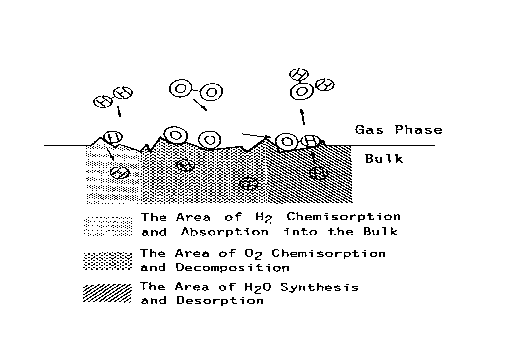 | |
Figure 2. Model of water synthesis process [12] |
Experimental technique
To perform the designed catalytic water synthesis reactions on platinum catalyst, an adequately equipped vacuum chamber was used. Schematic illustration of the experimental tribological system is depicted in Figure 3.
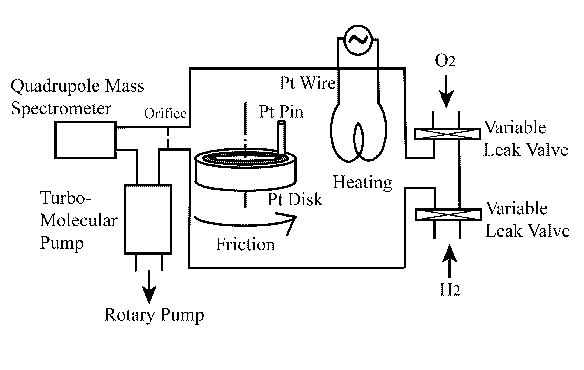 | |
Figure 3. Experimental set-up |
The system consists of two sections at different vacuum level. The section including a pin-on-disk tribometer is kept at the vacuum level of ~10-2 to 10-1 Pa. The second, high vacuum section (~10-5 Pa), is connected with a quadrupole mass spectrometer and two vacuum pumps: (i) rotary pump and (ii) turbo-molecular pump. Both sections are connected with the 1 mm diameter orifice. Hydrogen and oxygen are introduced into the first section chamber via a variable leak valve, and the desired gas pressure was obtained in the chamber by balancing the inlet and outlet of the gas. Exact partial pressure of each gas was determined in the second chamber section through the quadrupole mass spectrometer with a 1 s data sampling time. Purity of both gases was 99.99%. The end surface of the platinum pin (2 mm diameter) was slid against the rotating platinum disk (23 mm diameter). The purity of platinum was 99.99%. Both platinum specimens were polished with 600 grade emery paper. The procedure for determining the gas consumption during wear was based on the technique developed by Mori [13]. Detailed description and application of that procedure for similar tests along with some schematic illustrations can be found in paper [12].
The same vacuum chamber system was applied to observe the water synthesis process without friction. In this case the catalyst was used in the form of Pt 0.7 mm x 100 mm wire. It is to mention that under static conditions at ambient temperature none of these two catalyst types provided any noticed reactions leading to water synthesis. To initiate the catalytic reaction under static conditions electric heating was used in the first vacuum chamber. Figure 4 shows a picture of the test apparatus used for both static and dynamic experiments of platinum catalyzed water synthesis.
 | |
Figure 4. Test apparatus used for water synthesis experiments |
Heating test results
Left part of Figure 5 demonstrates the ion current change of hydrogen, oxygen and water during the platinum wire heating under static conditions. It is clearly seen that the first change relates to hydrogen desorption with the onset at the 1.25 minute heating time. Further temperature growth increases the hydrogen desorption, however, without any partial pressure changes in oxygen and water. Then, after some 40 seconds the oxygen partial pressure drops significantly, followed by dramatic hydrogen partial pressure decrease. At that time the water partial pressure appears. These findings are very interesting but not fully understood. The thermal desorption spectrum is clear and it reflects the binding energy of the absorbed hydrogen in platinum that is known to be a good accumulator for hydrogen somewhat similar to palladium. It was not possible to measure the lowest temperature desorption.
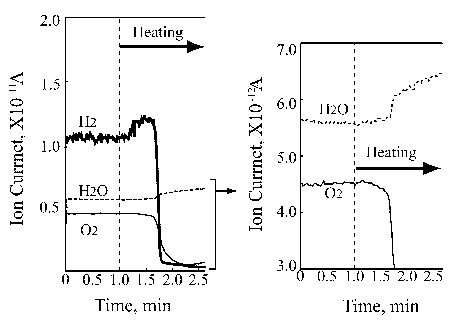 | |
Figure 5. Ion current change of hydrogen, oxygen and water in the platinum |
Referring to work [14] investigating temperature programmed desorption of hydrogen from different platinum crystal surfaces it is reasonable to estimate that temperature for ~100°C. However, it is also to note that experiments described in [14] were carried out for thin hydrogen adsorbed film ranging from 0.5 L to 50 L. On the other hand, it is clear that the hydrogen partial pressure dropped at the onset of water synthesis. Looking at the oxygen curve decrease one can assume that only a part of it was consumed for water synthesis leaving some oxygen chemisorbed on the platinum wire surface.
The right side of Figure 5 shows that the water synthesis starts immediately after the oxygen partial pressure drops. That means that the chemisorbed oxygen immediately reacts with the hydrogen available in the platinum wire catalyst.
Figure 6 depicts the ion current change of both substrates and the produced water during the 30 minute lasting heating of the wire. It is of importance to mention again that the hydrogen evolution commenced at the temperature range of around 100°C, however, the water synthesis began at much higher temperature of about 1000°C (the wire color was red). Figure 7 summarizes the heating test results.
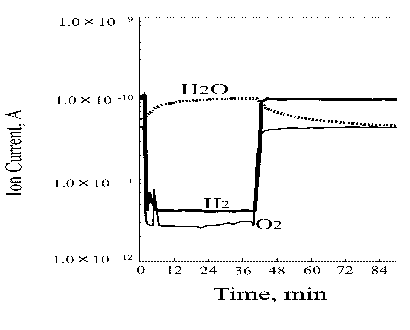 | |
Figure 6. Ion current change of substrates and the produced water during the wire heating process |
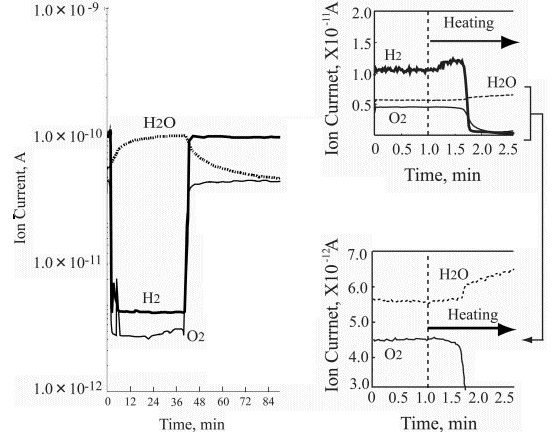 | |
Figure 7. Summary of the heating test results |
Tribological test results
Figure 8 presents the variation of ion current for each gas during the friction process of Pt/Pt triboelements (pin-on-disk system); the right figure side shows precisely measured water ion current versus the test duration. The pressure change of hydrogen and oxygen during the first test hour is very interesting and specific. Under sliding conditions oxygen is adsorbed/chemisorbed immediately and hydrogen evolution commences with no delay. The water synthesis process is noted immediately after the rubbing was started. The hydrogen desorbs very irregularly showing several maximums and minimums. The oxygen adsorption/chemisorption process is also irregular, however, there is no relationship with the hydrogen evolution process but one point in which the minimum hydrogen pressure corresponds to a small increase in the oxygen pressure. This test period is characterized by that the water partial pressure after the first increase at the test start is kept on the same level (see Figure 8). In this test period two findings should be emphasized. The first finding relates to very significant hydrogen evolution during the sliding. The second one is that only during this period of the friction test clear evidence for water synthesis can be seen.
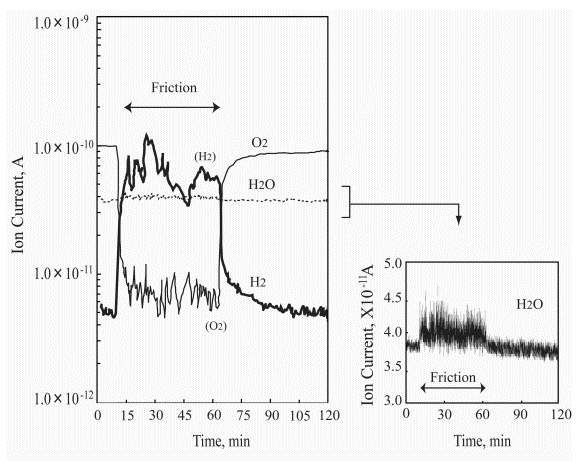 | |
Figure 8. Ion current for each gas during the friction process of Pt/Pt triboelements in the pin-on-disk system |
Next part of the ion current curves in Figure 8 is much more uniform. The hydrogen partial pressure decreases and the oxygen partial pressure increases. From that moment the water partial pressure is on a lower and stable level. The friction test was performed at the load of 2 N and 12 mm/s sliding velocity.
Discussion
Figure 9 illustrates the friction load influence on the water desorption/formation rate and oxygen chemisorption as a function of the applied load. It is evident that the water desorption and oxygen chemisorption rates increase with the increasing friction load.
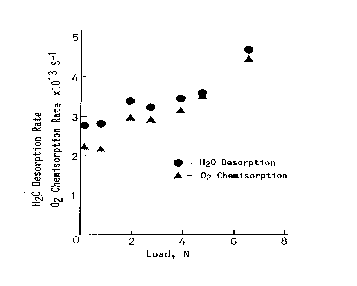 | |
Figure 9. Load influence on the water desorption/formation rate and oxygen chemisorption [12] |
This increase in both rates is due to the area increase of the generated fresh surface. However, as shown in work [12], the extrapolation of these plots to zero friction load does not lead to the origin situation. This evidences that even very low friction reveals clear catalytic effect of the platinum friction components.
When adsorbed atoms or molecules on a substrate are resistively heated, the surface species may desorb. This is because their surface residence time depends exponentially on temperature {t = to exp(DE/RT)}. Usually, if the adsorbate is not resupplied from the gas phase, its surface concentration diminishes rapidly with increasing temperature until the surface becomes clean. This phenomenon is the basis of temperature programmed desorption [3]. Maximum desorption occurs at a given temperature. In Figure 5 the upper curve presenting hydrogen desorption shows a broad maximum at the temperature range of about 100 to 800°C. This kind of desorption provides evidence that the platinum wire kept for at least 20 minutes in the hydrogen/oxygen mixture absorbs significant amount of hydrogen. Therefore, the chemisorbed oxygen molecules react very fast with hydrogen atoms available in the platinum wire. Figure 6 depicts well the water synthesis process.
To demonstrate the importance of hydrogen absorbed in the platinum wire for water synthesis experiments were performed using different oxygen initial pressures. The obtained results are extremely interesting. As Figure 10 shows, when the ion current of hydrogen did not change, more water was produced. A little bit higher water amount was obtained when I(O2) / I(H2) was about 0.8.
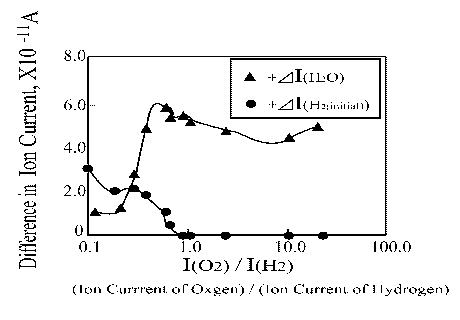 | |
Figure 10. H2O and H2 ion current change as a function of O2/H2 ratio |
This specific finding can be reflected in another form as depicted in Figure 11. In this case one can clearly see that when the hydrogen ion current was 1x10 -11 A, the water evolution showed highest.
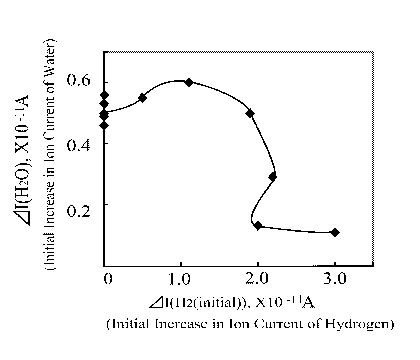 | |
Figure 11. Water ion current as a function of H2 initial ion current increase |
Figure 12 shows that the maximum increase in ion current by water desorption is obtained at the same I(O2)/I(H2) ratio as the maximum decrease in ion current by hydrogen and oxygen adsorption is obtained. Thus, Figure 12 demonstrates the most important finding concerning the thermal initiation reaction of the platinum catalyzed water synthesis reaction via hydrogen oxidation.
Considering the fact that in the applied research apparatus without heating platinum catalyzed water synthesis from H2 and O2 was not achieved and bearing in mind the fact that just by dropping a high-surface-area platinum gauze into the hydrogen and oxygen mixture the reaction occurs instantaneously, one significant question arises. The question to be asked is that. How this catalytic reaction is triggered and what reactive intermediates are responsible for the reaction? To get some necessary information to answer this question, Pt catalyzed water synthesis from hydrogen and oxygen was investigated under friction conditions.
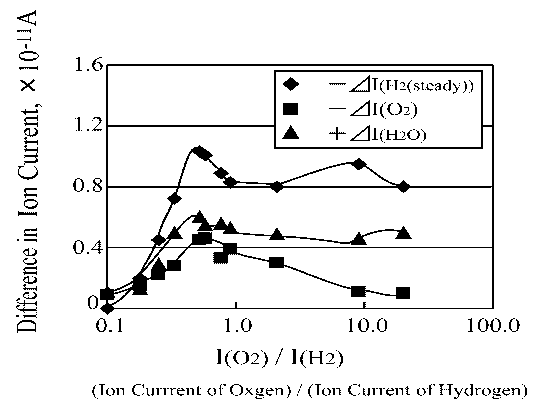 | |
Figure 12. H2, O2 and H2O ion current change as a function of O2/H2\ ratio |
First important information relates to the fact that also in the case of platinum friction elements being under similar temperature plus O2 and H2 partial pressure conditions, no water synthesis reaction was observed under static conditions. However, when the friction process was commenced, the water synthesis reaction began almost immediately with hydrogen evolution. It is to note at this point that the first noticed process relates to the oxygen chemisorption. When the friction process ceased no more water evolution was recorded. At the same time the H2 partial pressure dropped by one order of magnitude and the O2 partial pressure increased significantly. Both curves presenting the (i) hydrogen partial pressure decrease and (ii) oxygen partial pressure increase, are exponential (see Figure 8). The sudden hydrogen decrease clearly evidences why it was evolved when the friction process commenced. Platinum is known to absorb and diffuse hydrogen in it. The diffused hydrogen molecules in platinum mostly exist in atomic form (free radicals), as depicted in Figure 2. Accordingly, it is justified to suggest that most water is synthesized from the absorbed/diffused hydrogen radicals (atoms) and the chemisorbed oxygen. However, at this point the next question has to be asked. What is the reaction mechanism by which water is catalytically synthesized from hydrogen and oxygen when the process is initiated (i) by mechanical action (Pt pin sliding on Pt disk) and (ii) by heat (Pt wire electric heating)?
Earlier work [12] reported that the synthesis reaction of water from hydrogen and oxygen is enhanced by friction between platinum and platinum. To account for the obtained results the model of reaction process, as presented in Figure 2, was proposed. It was suggested that the worn surface consists of three active site types that provide three different functions: (i) the area where chemisorbed hydrogen is absorbed into the bulk, (ii) the area where chemisorbed oxygen is decomposed to the atoms, and (iii) the area where the chemisorbed oxygen and adsorbed hydrogen react, and water is desorbed. It was supposed that all these active sites are formed instantaneously and the reactions occur successively at different sites. It was assumed that the chemisorption rate of hydrogen is much less than the desorption rate of water. The enhancement of this reaction was assigned to the adhesive wear process. To account for all the findings it is necessary to elaborate a detailed reaction mechanism by which the tribocatalytic reaction process proceeds.
Proposed reaction mechanism
The proposed reaction mechanism is based on the fact that most of the hydrogen absorbed in platinum exists in the atomic form. On the other hand, it is taken into account that under friction conditions low-energy electrons are emitted. Water has a standard free energy of formation DG298 = -233 kJ/mol. Thus O2 and H2 gas mixtures may be stored indefinitely in a glass bulb without showing sign of any chemical reaction.
Our experiments demonstrate that also in the presence of either platinum wire or platinum pin/disk friction elements, no water production is observed without heating of the platinum wire or without friction process of the pin-on-disk elements. The obtained results clearly demonstrated that the driving force for the water synthesis reaction catalyzed by platinum wire relates to the thermal energy input and in the second case to the mechanical energy input. Figures 7 and 8 clearly demonstrate that the water synthesis process initiated by heat is definitely different from that proceeding under the friction process. One reason for that can concern different shape of the catalyst. However, the most important difference in both processes relates to the fact that under friction conditions the reaction is initiated immediately.
The following reaction mechanism is proposed for the tribocatalytic process. As Figure 13 demonstrates the first phase relating to static adsorption/chemisorption/diffusion processes of oxygen and hydrogen. Then, after the friction process onset low-energy electrons are emitted. One oxygen molecule attaches one electron and the oxygen negative-ion-radical reactive species is produced. According to Figure 14 the negatively charged end of the oxygen molecule interacts with the positively charged platinum surface spot left by the emitted electron and thereby the oxygen is chemisorbed. The other oxygen atom (free radical) looks for hydrogen atom (free radical) but not for hydrogen molecules (H2) that are available in the chamber. Hydrogen in the form of free radical is desorbed from the platinum disk wear track and interacting with the oxygen free radical forms hydroxyl group. Further reaction steps to form water are depicted in Figure 14.
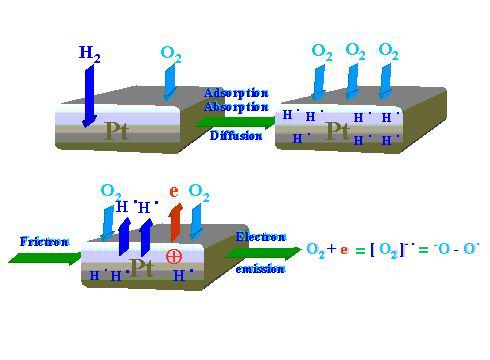 | |
Figure 13. First sequences of the water synthesis process during Pt pin sliding on Pt disk: interaction of hydrogen and oxygen molecules with the catalyst under static conditions and the electron emission phenomenon plus formation of the oxygen negative-ion-radical reactive species associated with friction |
Figure 13 shows that in the first dynamic stage hydrogen radicals H• and electrons are evolved. The emitted electron can easily interact with oxygen
molecule (O2) to produce radical-negative-ion (•O-O-). Then, as Figure 14 shows, the generated radical-anion reactive species looks for adequate positively charged site on the platinum surface where hydrogen radicals are available. Such interaction results in the formation of platinum bonded peroxyhydroxide. Peroxides are known to be very unstable due to the weak oxygen-oxygen bonding. Decomposition of the peroxide leads to generation of hydroxy-radical (HO•) just in the place where hydrogen free radicals are evolved (H•). Recombination of the two radical types produce water molecules.
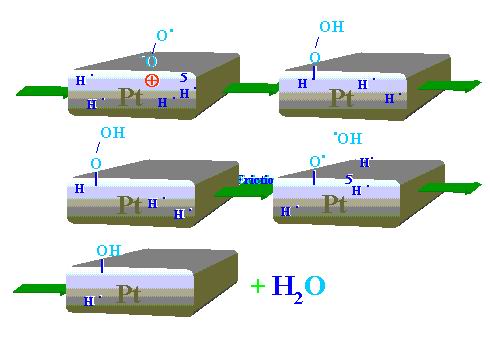 | |
Figure 14. Sequence of the tribocatalyzed water synthesis process during Pt pin sliding on Pt disk |
Conclusions
Based on the hypothesis of the present work, the mechanical action and separately only pure heat action on the platinum catalyzed water synthesis process were investigated. UHV experiments have been performed to generate adequate results for comparison of the effect of mechanically treated platinum catalyst with the thermally treated catalyst.
On the basis of the obtained results a new reaction mechanism is proposed. This specific mechanism is based on the fact that most of the hydrogen absorbed in platinum exists in the atomic form. On the other hand, it was taken into account that under friction conditions low-energy electrons are emitted. The proposed new reaction mechanism of water synthesis evidences the hypothesis of this work. To provide more evidence for the proposed mechanism, further research is planned combined with measurement of triboelectrons.
| |
References
- [1]
- G. HEINICKE: Tribochemistry. Akademie Verlag, Berlin (1984).
- [2]
- C.KAJDAS: Tribochemistry. In: Tribology 2001, 2nd World Tribology Congress, Eds. F. Franek. W.J. Bartz. A. Pauschtz. The Austrian Tribology Society, Vienna (2001), 39.
- [3]
- G.A SOMORJAI: Introduction to Surface Chemistry and Catalysis. John Wiley & Sons, New York (1994).
- [4]
- C. KAJDAS: Importance of Anionic Reactive Intermediates for Lubricant Component Reactions with Friction Surfaces. Lubrication Science, 6 (1994), 203.
- [5]
- C. KAJDAS: A Novel Approach to Tribochemical Reactions: Generalized NIRAM-HSAB Action Mechanism. Proc. International Tribology Conference, Yokohama 1995, Satellite Forum on Tribochemistry. JST, Tokyo (1995), 31.
- [6]
- G.W. KOEPPL: Best ab initio Surface Transition State Theory Rate Constants for the D+H2 and H+D2 Reactions. J. Chem. Phys. 59 (1973) 3425.
- [7]
- M. SALMERON, R.J. GALE, G.A. SAMORJAI: A Modulated Molecular Beam Study of the Mechanism of the H2-D2 Exchange Reaction on Pt(111) and Pt(332) Crystal Surfaces. J. Chem. Phys. 70 (1979) 2807.
- [8]
- H. BONZEL, G. BRODEN, G. PIRUG: Structure Sensitivity of NO Adsorption on a Smooth and Stepped Pt(100) Surface. J. Catal. 53 (1978) 96.
- [9]
- T. SASADA, K. HIRATSUKA, H.SAITO: Adsorption of Surrounding Gas Molecules on Pure Metal Surfaces During Wear Processes. Wear 135 (1990) 251.
- [10]
- M. KUZUYA, K. HIRATSUKA, T. SASADA: Friction Catalysis -1st report- Proc. 33rd JSLE Annual Meeting, December (1988) 581, in Japanese.
- [11]
- M. KUZUYA, K. HIRATSUKA, T. SASADA: Friction Catalysis -2nd report- Proc. 33rd JSLE Annual Meeting, May (1989) 321, in Japanese.
- [12]
- K. HIRATSUKA, M. KUZUYA, T. SASADA: Friction Catalysis in the Synthesis of H2O during Adhesive Wear. Proc. 33rd Japan Congress on Materials Research, Kyoto, Japan 1990, 191.
- [13]
- S. MORI, M. SUGINOYA, Y. TAMAI: Chemisorption of Organic Compounds on a Clean Aluminum Surface Prepared by Cutting under High Vacuum. ASLE Trans. 25 2 (1982) 261-266.
- [14]
- G.A. SOMORJAI: Chemistry in Two Dimensions: Surfaces. Cornell University Press, Ithaca, N.Y. 1981.
| |













