| |
Investigation of tribochemical behavior of Al-Si alloy against itself lubricated by amines
Lilian Hua, Jianmin Chena, Weimin Liua, *, Qunji Xiiea Czeslaw Kajdasb
aLaboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, The Chinese Academy of Sciences, Lanzhou 730000, PR China
bInstitute of Chemistry, Warsaw Technical University in Plock, 00-400 Plock Poland
Keywords: Amines; Al-Si alloy; Friction and wear behaviors; XPS and FT-IR analyses
Published in : Wear 243 (2000) 60-67
*Corresponding author. Fax: +86-931-827-7088.
Abstract
The friction and wear properties of Al-Si alloy against Al-Si alloy was investigated using an Optimol SRV Tester with the lubrication of pure ethyleneglycol, ethanolamine, ethylenediamine and triethylenetetramine. The tribo-chemical reactions and the compositions of the boundary film formed on the rubbed surface of Al-Si alloy were examined by X-ray Photoelectron Spectrometer (XPS). The wear debris of Al-Si with the lubrication of triethylenetetramine was collected and evaluated with FT-IR, and thermal gravimetric (TG) analysis.
Friction and wear tests reveal that triethylenetetramine shows the best antiwear capabilities among the tested compounds. The results of FT-IR, TG and XPS indicate the occurrence of tribo-chemical reactions between Al and amine with the formation of a chemically stable complex of aluminum and amine, as well as the formation of friction polymer.
1. Introduction
Al-Si alloys have the potential to be used in the tribological applications such as internal combustion engines, plain bearings, compressor and refrigerator. Although Al-Si alloys meet many of the requirements for such applications, they are vulnerable in the sliding process. They are difficult to be lubricated and exhibit poor resistance to damage. The tribological properties of Al-Si alloy under dry friction condition have been extensively investigated [1-10]. In order to improve the friction and wear properties of Al-Si alloy, some self-lubricating Al-Si composites which contain solid lubricants such as graphite, molybdenum disulfide and soft metals have been prepared [11-15]. Investigations of Al-Si alloy against steel with engine oils or polyalkyleneglycols have shown that none of the lubricants were as effective as they were in steel-on-steel system [16-118].
In boundary lubrication of aluminum, the chemical interaction, especially the chemical reaction of a lubricant with the rubbing surfaces is very important. Tests have indicated that the conventional S, P, Cl antiwear additives are not very effective in preventing the seizure of aluminum [19-20]. Some researchers have investigated the lubricity of alcohol [21-22], partial ester [23] and diol [24-25] for aluminum alloy. According to the researches reported on organometallic compounds, some organic ligands with rich electrons such as N and 0 may interact with the aluminum to form a stable aluminum complex [26]. It is speculated that the complexes formed in the boundary film during sliding process can prevent seizure of aluminum and to reduce friction and wear of the sliding system.
In this paper, the friction and wear properties of Al-Si alloy lubricated with pure ethyleneglycol, elhanolamine, ethylenediamine and triethylenetetramine were evaluated. The tribo-chemical reactions between Al-Si and lubricants were investigated in an attempt to understand the reaction mechanism of additives for lubrication of aluminum.
2. Experimental details
The tested additives, ethyleneglycol, ethanolamine, ethylenediamine and triethylenetetramine are all commercially chemical reagents without purification before utilization. The formula and molecular weight of these compounds are listed in Table 1. The aims of selection of ethyleneglycol, ethanolamine, ethylenediamine and triethylenetetramine were to investigate the antiwear function of organic groups such as —NH2, —OH, and the combination of —NH2 and —OH.
Table 1 The formula and molecular weight of the
selected compounds | |
Compounds | Formula | Molecular
weight | Purity
(%) | |
Ethylenediamine | H2NCH2NH2 | 60 | 98 | |
Ethyleneglycol | HOCH2CH2OH | 62 | 98 | |
Ethanolamine | H2NCH2CH2OH | 61 | 98 | |
Triethylene- tetramine | H2NCH2CH2NHCH2 | 146 | 98 | |
CH2NHCH2CH2NH2 | 146 | 98
|
Friction and wear tests were conducted on an Optimol SRV Tester, using a sliding of Al-Si cylinder with a 16.10mm diameter against an Al-Si block. Both the cylinders and the blocks were made of Al-Si alloy with a silicon content of 12wt.% and the Brinell hardness of 67.5kg mm -2. The surface roughness of the specimens was about Ra 0.08 µm. Before each test, the specimens were cleaned with petroleum ether. The SRV apparatus used has been described in [17,21] in detail. The test conditions were: frequency 20 Hz, amplitude 1 mm, test duration 15 min, room temperature. The lubricant, with a volume of 0.5 ml, was applied onto the contacting area before friction test. The friction coefficient was recorded continuously by the tester, and wear of the block was measured by sliding a profilometer across the wear track at the middle part of wear trace.
Since X-ray Photoelectron Spectrometer (XPS) is a very sensitive tool to investigate the chemical composition and chemical environment of the elements in a material, it was used to evaluate both the wear debris and the boundary film formed on the surface of Al-Si alloy. FT-IR is also a useful tool to study the organic group of a material, and was used in this work to analyze the wear debris of specimens lubricated with pure triethylenetetramine. A Bio-Rad FT-IR Spectrometer was used in the study. XPS analyses were conducted on a PHI-5702 electron spectrometer using pass energy of 29.35 eV and the Mg Ka line excitation source with the reference ofCis at 284.6 eV. The fitting curves were conducted by the software of PC Access ESCA Version 6.0 in the work station of HP-XU 6/150 of Perkin-Elmer. In order to understand the chemical behavior of the wear debris, a TG analysis was conducted under N3 on a PE-7 Series Thermal Analyses System using a heating rate of 10 °C/min.
3. Test results
3.1. Friction and wear results
Fig. 1 shows the friction coefficient as a function of testing time under the load of SON for ethyleneglycol, ethylenediamine, ethanolamine and triethylenetetramine, respectively. Results indicate that the friction coefficient for three of the fluids tested is quite close, ranging from 0.29 for ethyleneglycol to 0.27 for ethanolamine and ethylenediamine. The lowest friction coefficient is observed for triethylenetetramine, which had a friction coefficient ranging from 0.24 to 0.16.
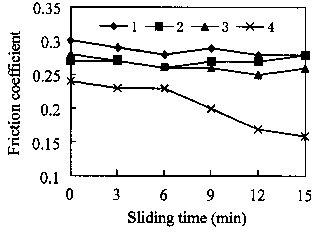 |
Fig. 1. Friction coefficient as a function of sliding time with the lubrication of (1) ethyleneglycol; (2) ethylenediamine; (3) ethanolamine; and (4) triethylenetetramine (SRV, cylinder-on-block, 20 Hz, 1 mm, 30 N, 15min, 25°C).
|
The profiles of the wear tracks of the Al-Si blocks are founded in Fig. 2. The data were obtained from the middle part of the wear tracks after l5min tests under the load of ION. Results in Fig. 2 indicate that the wear of the block lubricated with ethyleneglycol is the largest. The wear of block lubricated with ethanolamine and ethylenediamine are about the same, and the lowest wear occurred in the system lubricated with triethylenetetramine. A rough calculation from the areas of curves 1 and 4 covered shows that the wear of the block lubricated with ethyleneglycol is about three times higher compared to triethylenetetramine.
3.2. FT-IR and TG analyses
In order to investigate the tribochemical reactions between triethylenetetramine and Al-Si alloy, a friction test was conducted using triethylenetetramine. The load was at 200 N, test duration was 60 min, the lubricant with volume of 0.5 ml was applied onto the contacting area before test, and the wear debris was collected.
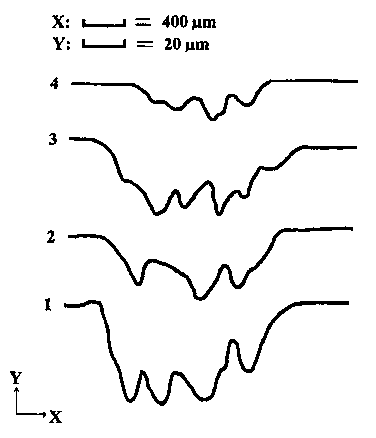 |
Fig. 2. Profiles of wear track of Al-Si block with the lubrication of (1) ethyleneglycol; (2) ethylenediamine; (3) ethanolamine and (4) triethylenetetramine (SRV, cylinder-on-block, 20 Hz, 1mm, ION, l5min, 25°C).
|
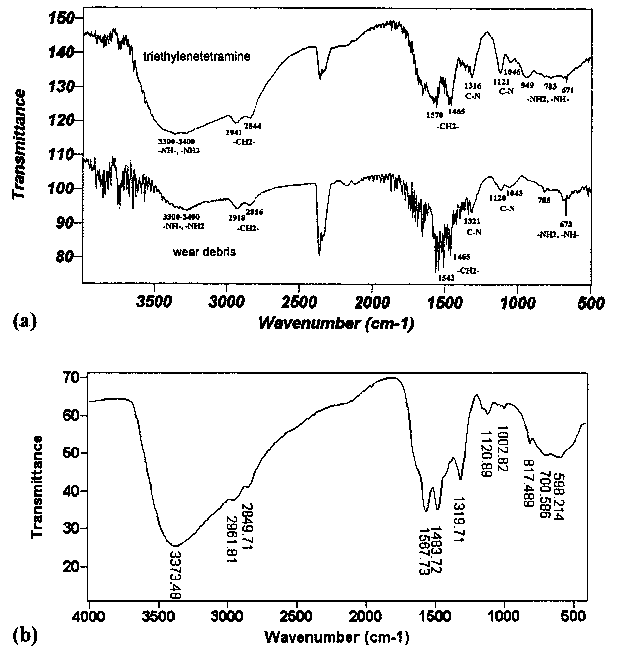 |
Fig. 3. IR spectra of
(a) pure triethylenetetramine and wear debris of Al-Si lubricated with pure triefhylenetrtramine under load of 200 N at 25°C for 60 min and
(b) triethylenetrtramine after sliding under load of 200 N at 25°C for 60 min.
|
The collected wear debris was washed with petroleum ether twice to remove the unreacted triethylenetetramine and analyzed by FT-IR. As a comparison, the pure triethylenetetramine before and after test were also analyzed with FT-IR. The results are shown in Fig. 3. Results reveal that the lubricant before and after sliding test is quite similar, may be because of the change of triethylenetetramine was too small to be detected. From the result of wear debris, it was found that the intensity of wavenumbers at 3300-3400 and 670-950 cm-1 of —NH2 group reduced remarkably, possible indicating the interaction via -NH2 to Al-Si alloy.
Three thermal weight loss tests of (a) the collected wear debris; (b) the combination of triethylenetetramine and Al-Si powder with weight ratio about 1:1; and (c) the triethylenetetramine after friction test; were carried out with PE-7 Series Thermal Analyses System at the heating rate of 10 °C min-1 under N2 atmosphere. Results in Fig. 4 show that the combination of triethylenetetramine and Al-Si powder with weight ratio about 1; 1 lost weight gradually at the start of the test, and it lost about 47% of the total weight of the mixture at a temperature around 170 °C. It is interesting to point out that at 594 °C, there is a small weight increase (total about 2% at 800°C), which is believed to be the result of reaction between Al-Si and N2. The TG results of wear debris show that it lost about 14% weight from the start to the temperature of 200°C, but it still lost weight after 200 °C with an onset of 276°C, possibly indicating the breakage of the complex of Al-Si and amine or some kind of friction polymer, and the totally lost weight is about 27% of the whole wear debris. Quite similar to the results of Al-Si and amine mixture, the wear debris gets weight also after the temperature of 500°C with onset 576.7°C, and total increased weight is about 10% at 800°C. It is supposed that after the temperature ofTG test was 800°C, the left material was mostly inorganic compounds. Accordingly, at the TG test with the combination of triethylenetetramine and Al-Si powder at the weight ratio of 53:47, 2% weight increase was observed after the loss of organic compounds. For the TG test of wear debris, 73% of Al-Si totally gained 10% of weight increase. As a comparison, 10/73 (0.137) is higher than 2/47 (0.043). From the higher percentage of the weight increase after 500°C and the lower of the onset temperature of the reaction (577°C versus 594°C), it could be anticipated that the Al-Si in wear debris reacted easier with N2 than that of the powder of Al-Si which might be covered with a layer of aluminum oxide. During friction process, the triethylenetetramine may interact with Al-Si alloy to form some kind of organic complex. At high temperature under N2 atmosphere, this complex will decompose to gen
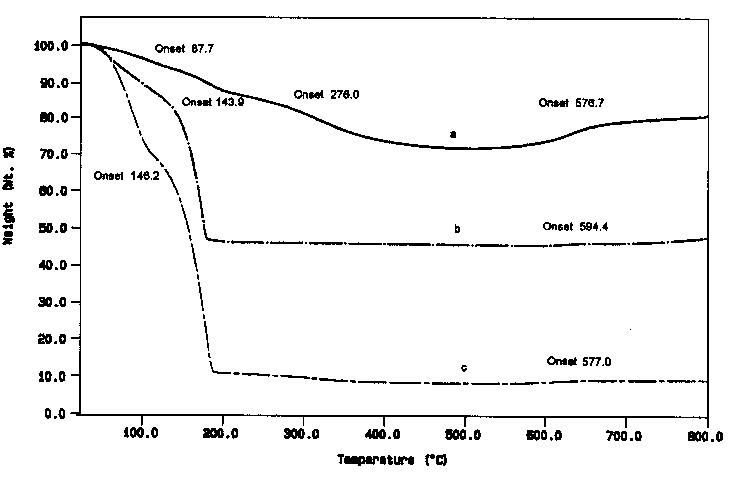 | |
Fig. 4. Results of TG analyses of (a) pure triethylenetetramine and aluminum powder with weight ratio of 1:1; (b) wear debris of Al-Si lubricated with pure triethylenetrtramine under load of 200 N at 25°C; and (c) triethylenetrtramine after sliding test under load of 200 N at 25°C for 60min (heating rate 10°C min-1, N2 atmosphere).
|
erate Al-Si with fresh surface, as a result, N2 will quite easily react with the fresh Al-Si to form a new compound - AlN. The Gibbs free energy of the following reaction is -287.8 kJ/mol, indicating the easy reaction of aluminum and nitrogen:
Al + ½N2 → AlN
However, the reaction of
Al2O3 + N2 → AlN + NO
is not possible until the temperature reaches 9114 K at which the Gibbs free energy will be negative. In XPS analyses, a small peak with the binding energy of Al2p at 71.0 eV could be detected, indicating even in air there is still some aluminum on the surface of Al-Si specimen in metal state but not in the form of aluminum oxide. This fact may explain why N2 can react with the powder of Al-Si under nitrogen atmosphere at temperature higher than 500°C.
Fig. 4 also indicates that there is no obvious difference of weight loss of curve (c) with curve (b) before temperature around 180°C, considering the percentage of triethylenetetramine in (b) test was only about half of it in (c) test. However, in (b) test, all triethylenetetramine decomposed and lost weight at about 180°C, while in (c) test, the tested lubricant after sliding at 200 N for 60 min decomposed and lost weight totally only at about 190°C. Such a 10°C difference may be corresponding to the decomposition of a kind of friction polymer formed during sliding test. Since in (c) test the lubricant contains small amount of wear debris (about 10%), similar curve as in (a) test after 190°C could be observed.
3.3. XPS analyses
XPS analyses usually give one curve of binding energy of an element. With the help of software of PC Access ESCA Version 6.0 of Perkin-Elmer, some curve can be fitted into several curves with different binding energies. As indicated in Fig. 5b, the top two curves are original and fitting curves, respectively, while the other three curves are related to three forms of nitrogen with different binding energies. If those top two curves are very close, it means that the fitting process is to be more accurate. Fig. 5 shows the binding energy of N1s of the wear debris before and after TG tests. Results in Fig. 6a illustrate that the binding energy of N1s in the wear debris is in three forms with the binding energies of 399.0, 399.8 and 401.1 eV. The binding energy of 399.0 eV corresponds to adsorbed forms of triethylenetetramine, whereas the binding energies of 399.8 and 401.1 eV may relate to degraded, oxidized or even polymer forms of compounds possible such as Al-NH-C- and -NH-CH2COO-, respectively [27]. A big variance was observed to the curve of N1s in wear debris after TG test under N2, as indicated in Fig. 6b. The results show that after TG test, a new curve with the binding energy of 397.8 eV could be observed, illustrating the formation of AlN. The other binding energies at 399.4 and 400.7 eV might be related to the residual of oxidized or degraded forms of triethylenetetramine.
XPS analyses were also carried out for elements of C, N, Al and Si on the rubbed surfaces of Al-Si alloy lubricated with pure triethylenetetramine, and all the given binding energies were shifted as the reference of C1s at 284.6 eV. Results in Fig. 6 show that the Al might be existing in three forms with the binding energies of 74.7, 73.8 and
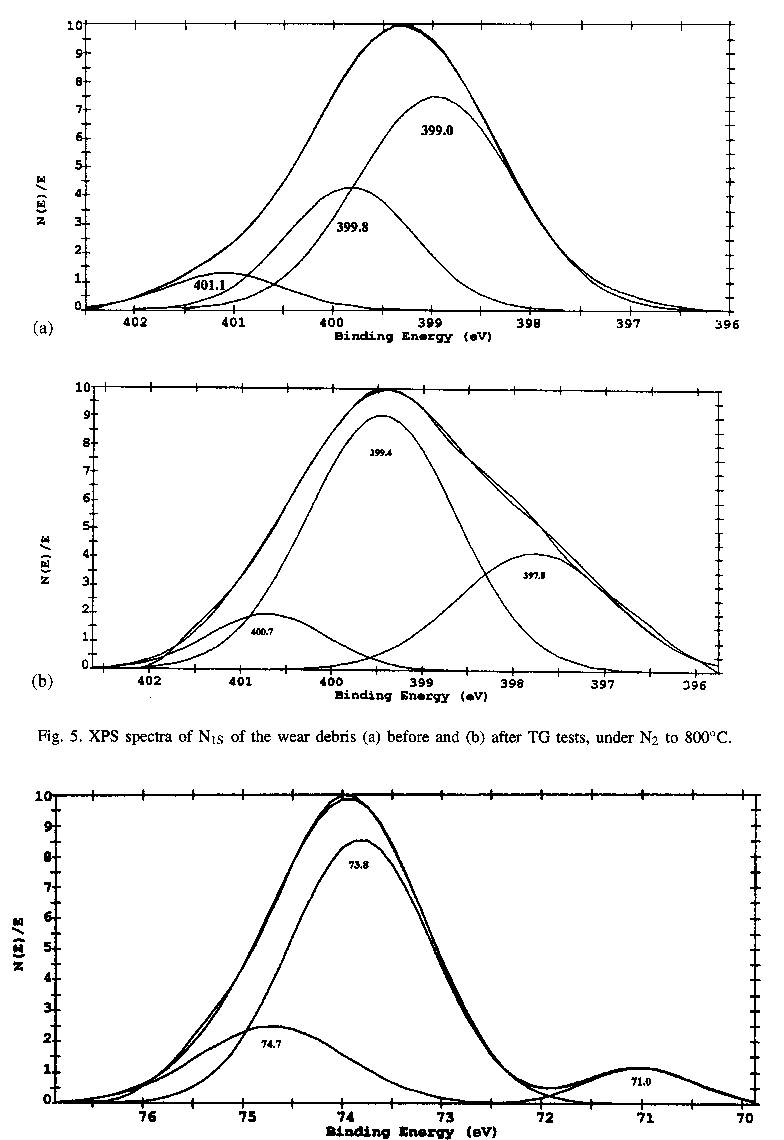 | |
Fig. 5. XPS spectra of N1s of the wear debris (a) before and (b) after TG tests, under N2 to 800°C.
|
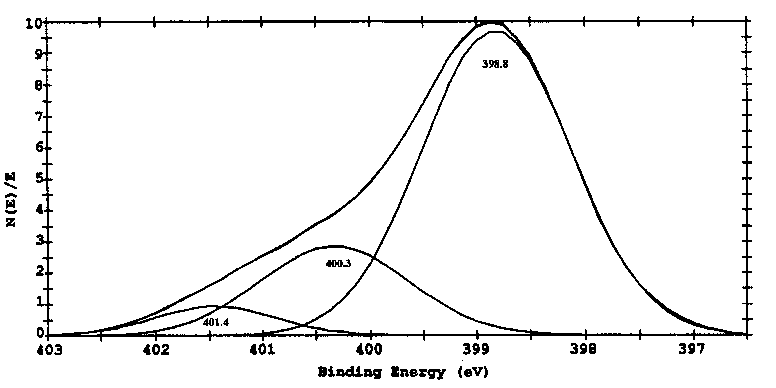 | |
Fig. 6. XPS spectra of AL2p on the boundary films after SRV test (SRV, cylinder-on-block, 20 Hz, 1 mm, SRV, 15min, 25°C).
|
72.0 eV, respectively. The binding energy of Al2p at about 74.7 eV is related to Al2O3 , and the binding energy of Al2p at about 72.0 eV is corresponding to Al-Si alloy. However, it is complicated for the binding energy at 73.8eV, most possible explanation is related to aluminum complexes with degraded or oxidized triethylenetetramine [27]. Results in Fig. 7 show that the binding energy of N1s in the boundary films is also in three forms with the binding energies of 398.8, 400.3 and 401.4 eV, indicating the adsorbed forms of triethylenetetramine and degraded, oxidized or even polymer forms of triethylenetetramine. The binding energy of N1s at 400.3 eV seems related to organic compound such as Al-NH-C-, whereas the binding energy of N1s at 401.4 eV may correspond to an organic compound in the form of -NH-CH2COO- The above results illustrate that some tribochemical reaction took place during sliding process.
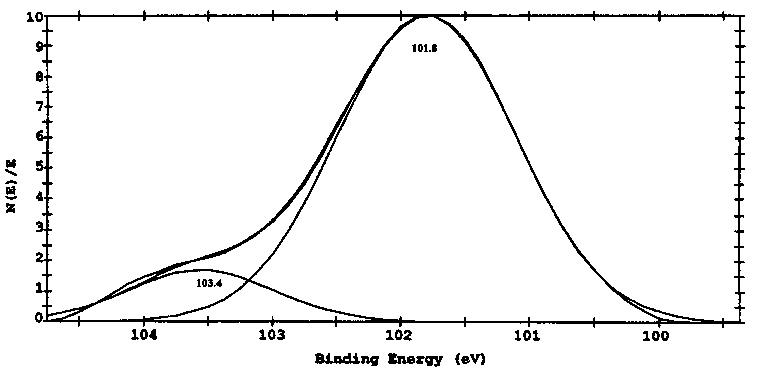 | |
Fig. 7. XPS spectra of N1s on the boundary films after SRV tests (SRV, cylinder-on-block, 20 Hz, 1 mm, 30 N, 15min, 25°C).
|
Figs. 8 and 9 show the curves of binding energies of Si2p and C1s of specimen lubricated with triethylenetetramine. Results in Fig. 8 indicate that the Si on the boundary film is both in the form of Al-Si alloy with the binding energy of 101.8eV and SiO2 with binding energy of 103.4 eV. Results in Fig. 9 show that the binding energy of C1s in the boundary films is in three forms with the binding energies of 284.5, 285.8 and 287.2 eV, indicating the adsorbed forms of triethylenetetramine and oxidized triethylenetetramine. The binding energy of C1s at 285.8 eV is related to organic group of -CO-, whereas the binding energy of C1s at 287.2 eV is corresponding to organic group of -COO- [27]. All these oxidized compounds interacted or even reacted with aluminum quite easily.
Table 2 summarizes the chemical compositions and chemical states of the boundary film lubricated by triethylenetetramine. According to the results in Figs. 6-9, elements C, N and Al all exist in three forms. Of the total nitrogen in the boundary film, the percentage of the adsorbed triethylenetetramine is about 73% with the binding energy of 398.8 eV, and the nitrogen in complex form is about 27% with the binding energies of 400.3 and 401.4eV, which possibly included -HN-CH2COOAl and Al-NH-CH2-CH2NH-
4. Discussion
In boundary lubrication of Al-Si on Al-Si, the chemical interaction especially the chemical reaction of a lubricant with the rubbing surfaces is very important. Hotten has investigated the lubricity of diol and ketol for aluminum, and has suggested that the antiwear mechanism is due to the formation of aluminum diol complex or aluminum ketol complex [24]. Investigations in Laboratory of Solid Lubrication, Chinese Academy of Sciences proved the formation of aluminum diol complex, and found that the complex
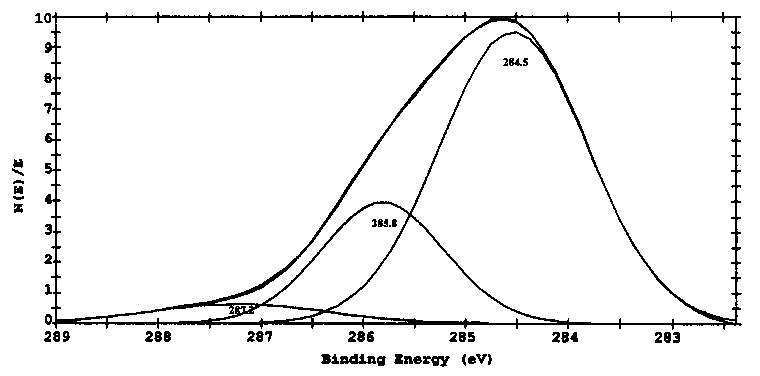 | |
Fig. 8. XPS spectra of Si2p on the boundary films after SRV tests (SRV, cylinder-on-block, 20 Hz, 1mm, SON, 15min, 25°C).
|
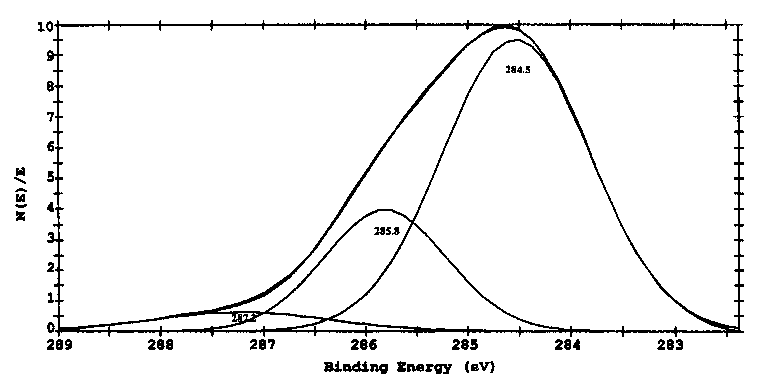 | |
Fig. 9. XPS spectra of Cis on the boundary films after SRV tests (SRV, cylindcr-on-block, 20 Hz, 1mm, 30 N, 15min, 25°C).
|
Table 2 The binding energy (BE, eV) and
atomic percentage of elements in boundary film
lubricated by triethylenetetramine at 30 N for 15min | |
C | N | Al | Si | |
BE | (%) | BE | (%) | BE | (%) | BE | (%) | |
287.2 | 5.4 | 401.4 | 5.6 | 74.7 | 21.8 | 103.6 | 12.2 | |
285.8 | 24.4 | 400.3 | 21.4 | 73.8 | 71.1 | 101.8 | 87.8 | |
284.5 | 70.2 | 398.8 | 73.0 | 71.0 | 7.1
|
of aluminum with 1,3-diol is more chemically stable than corresponding 1,4-diol or 2,3-diol [25]. Kajdas has put forward an anionic-radical concept to illustrate the lubrication mechanism of alcohol to aluminum [28]. The results in this work illustrate that triethylenetetramine, diamine and ethanolamine exhibit better performance to reduce wear of the Al-Si alloy, as compared to the ethyleneglycol. Among the tested four compounds, triethylenetetramine shows the lowest friction coefficient and smallest wear. Since there are two electrons which are not bonded in the atom of nitrogen in the amine-like compounds, it is quite easy to interact with metals. In triethylenetetramine, the percentage of N is smaller than that of in diamine, but it exhibits superior tribological performance to diamine. One explanation to this fact is that the molecular chain of triethylenetetramine is longer than diamine. Another but important one is related to the greater tendency of oxidation and easier interaction of the oxidized compounds with the Al-Si alloy. From the bond energy as listed in Table 3, it could be anticipated
Table 3
Bonds energy in
triethylenetetramine | |
Bonds | Bond energy
(Kcal mol-1) | |
C—H | 99.3 | |
N—H | 93.4 | |
C—C | 82.6 | |
C—N | 72.7
|
that in triethylenetetramine, C—N bond is the easiest bond to break. If triethylenetetramine oxidizes or degrades under air, possible products will include H2NCH2COOH and —NHCH2CH2NH2. Those compounds are liable to interact with the fresh surface of Al which is generated during sliding process, or to form some kinds of friction polymer. According to the results of XPS analyses, the binding energy of N1s at 400.3 eV seems related to organic compound such as Al—NH—C—, while the binding energy of N1s at 401.4eV may be corresponding to organic compound in the form of —NHCH2COOH—. From the binding energy of Al2p at 73.5eV, it can also be inferred that a complex of aluminum and organic compound was formed during friction process. Based on the results of TG, FT-IR and XPS analyses, it can be concluded that triethylenetetramine degraded or oxidized during sliding process with the formation of a complex between aluminum and degraded compounds, and possible some friction polymers.
5. Conclusions
- As tested, triethylenetetramine shows the best antiwear ability in an Al-Si against Al-Si system. Among ethyleneglycol, ethanolamine, ethylenediamine and triethylenetetramine, ethanolamine and ethylenediamine exhibit better performance than ethyleneglycol.
- Surface analyses of the rubbed surface of Al-Si alloy suggest the interaction between Al-Si alloy and triethylenetetramine. The analyses performed suggest the formation of an aluminum and amine complex such as H2N—CH2COOAl and Al—NH—CH2—CH2NH2, or some kinds of friction polymer.
- The wear debris of Al-Si alloy, collected from wear test lubricated with triethylenetetramine, reacted easier with N2 to form AlN during TG test, as compared to the powder of Al-Si during TG test under N2 atmosphere. This result indicates that a fresh surface of wear debris of Al-Si was generated during thermal weight test, due to the breakage of aluminum and amine complex at high temperature.
Acknowledgements
Senior Engineer Shangkui Qi at Laboratory of Solid Lubrication of Lanzhou Institute of Chemical Physics is gratefully acknowledged for performing the XPS analyses. The authors also wish to acknowledge the financial support of National Natural Science Foundation of China and the Ministry of Science and Technology of China.
| |
References
- [1]
- 1 A.D. Sarkar, J. Clarke, Friction and wear of aluminum-silicon alloys, Wear 61 (1980) 157-167.
- [2]
- C. Subramanian, Effects of sliding speed on the unlubricated wear behaviour of Al-12.3 wt.% Si alloy. Wear 151 (1991) 97-110.
- [3]
- S. Das, B.K. Prasad, Tribological behaviour of aluminum alloy composites: a comparative study with a copper-based alloy, Wear 162/164 (1993) 64-74.
- [4]
- H. Torabian, J.P. Pathak, S.N. Tiwari, Wear characteristics of Al-Si alloys. Wear 172 (1994) 49-58.
- [5]
- K.M. Jasim, E.S. Dwarakadasa, Wear in Al-Si alloys under dry sliding conditions. Wear 119 (1987) 119-130.
- [6]
- C. Subramanian, On mechanical mixing during dry sliding of aluminum-12.3 wt.% silicon alloy against copper, Wear 161 (1993)
53-60.
- [7]
- A. Somi Reddy, B.N. Pramila Bai, K.S.S. Murthy, S.K. Biswas, Wear and seizure of binary Al-Si alloys. Wear 171 (1994) 115-127.
- [8]
- H.A. Hanna, F. Shehata, Friction and wear of Al-Si alloys, Lubrication Eng. 49 (1993) 473-176.
- [9]
- D.P. Mondal, S. Das, B.K. Prasad, Study of erosive-corrosive wear characteristics of an aluminium alloy composite through factorial design of experiments. Wear 217 (1998) 1-6.
- [10]
- H. Akbulut, M. Durman, F. Yilmaz, Dry wear and friction properties of Al2O3 short fiber reinforced Al-Si (LM13) alloy metal matrix composites. Wear 215 (1998) 170-179.
- [11]
- S. Das, S.V. Prasad, T.R. Ramachandran, Microstructure and wear of cast (Al-Si alloy)-graphite composites. Wear 133 (1989) 173-188.
- [12]
- PR. Gibson, A.J. Clegg, A.A. Das, Wear of cast aluminum silicon alloys containing graphite, Wear 95 (1984) 193-198.
- [13]
- H. Torabian, J.P. Pathak, S.N. Tiwari, On wear characteristics of leaded aluminum-silicon alloys. Wear 177 (1994) 47-54.
- [14]
- J.P. Pathak, D. Karimi, S.N. Tiwari, Room temperature wear characteristics of Al-Si-Cd bearing alloys. Wear 170 (1993) 109-
117.
- [15]
- W.J. Tomlinson, A.S. Bransden, Cavitation erosion of laser surface alloyed coating on Al-12% Si, Wear 185 (1995) 59-66.
- [16]
- J. Jiang, A. Ma, H. Liu, R. Tan, The wear properties of an alumina-aluminosilicate fiber hybrid reinforced Al-Si alloy in a
lubricated condition, Wear 171 (1994) 163-168.
- [17]
- Q. Wang, H.S. Cheng, M.E. Fine, Wear of tin coating and Al-Si alloy substrate against carburized steel under mixed lubrication, Tribol. Trans. 37 (1994) 277-284.
- [18]
- S. Komatsuzaki, Y. Homma, K. Kawashima, Y. Itoh, Polyalklcne glycol as lubricant for HFC-134a compressors. Lubrication Eng. 47 (1991) 1018-1025.
- [19]
- Yong Wan, Qunji Xue, Effect of antiwear and extreme pressure additives on the wear of aluminum alloy in lubricated aluminum-on-steel contact, Tribol. Int. 28 (1995) 553-557.
- [20]
- Yanhong Hu, Weimin Liu, The effects of molecular structure of organo chlorine on the lubricity of Al 2024 against steel, Wear 218 (1998) 78-83.
- [21]
- R.S. Montgomery, The effects of alcohols and ethers on the wear behavior of aluminum. Wear 8 (1965) 466-473.
- [22]
- Yanhong Hu, Weimin Liu, Tribological properties of alcohols as the lubricating additives for aluminum-on-steel contact. Wear 218 (1998) 244-249.
- [23]
- S. Hironaka, T. Sakurai, The effect of pentaerythritol partial ester on the wear of aluminum. Wear 50 (1978) 105-114.
- [24]
- B.W. Hotten, Bidentato organic oxygen compounds as boundary lubricants for aluminum. Lubrication Eng. 30 (1974) 398-
403.
- [25]
- Yong Wan, Weimin Liu, Qunji Xue, Effects of diol compounds on the friction and wear of aluminum alloy in a lubricated
aluminum-on-steel contact. Wear 193 (1996) 99-104.
- [26]
- Weimin Liu, Yanhong Hu, Zhiming He, Pingyu Zhang, Qunji Xue, Friction and wear behaviour of an Al-Si alloy against steel lubricated with N- and O-containing organic compounds, Lubrication Sci. 11 (1998) 37-49.
- [27]
- C.D. Wagner, W.M. Riggs, L.E. Davis, J.F. Moulder, G.E. Muilenberg, Handbook of X-Ray Photoelectron Spectroscopy,
Perkin-Elmer Corporation, 1978.
- [28]
- C. Kajdas, About an anionic-radical concept of the lubrication mechanism of alcohols, Wear 116 (1987) 167-180.
| |








