| |
WEAR AND CHEMICAL REACTIONS
KEN`ICHI HIRATSUKA*,1, CZESŁAW KAJDAS 2
1 Department of Mechanical Science and Engineering, Chiba Institute of Technology, Chiba
2 Institute of Chemistry, Warsaw University of Technology, Plock
Abstract
The relation between adhesive wear mechanism and chemical reactions has been reviewed from the viewpoint of tribofilm formation and processing time. In severe wear mode, after the transfer particle grew at the interface of two sliding materials, it was separated from the interface. Then it was called wear particle. This process was enhanced when the sliding system occurred in oxygen atmosphere. Environmental oxygen around the contact region enhanced the growth process of transfer particles (tribofilm). On the other hand, environmental oxygen around the non-contact region increased the amount of wear by the logarithmic function of the interval time. Severe-mild wear transition observed in the rubbing between copper and iron has been studied in detail. The sliding distance needed for the transition increased with increasing sliding velocity and applied load. It is deduced that severe-mild wear transition appears after a certain time has elapsed and tribofilm of adequate thickness has grown to protect the surface. The time is also determined by the applied load. The tribochemical reactions are enhanced by wear through the exo-electron emission and explained by NIRAM (Negative-Ion-Radical-Action-Mechanism) approach. The reaction cycle is proposed in the sequence: wear ® exo-electron emission ® chemical reaction ® tribofilm formation ® wear…. This explains the complex phenomena associated with wear and chemical reactions.
Keywords: adhesive wear, chemical reaction, severe wear, mild wear, NIRAM, exo-electron emission
* Corresponding author: E-mail: hiratsuka @ pf.it-chiba.ac.jp
1. Introduction
It has been widely accepted that the forms of wear are classified into four distinct ones listed below, which were originally proposed by Burwell [1]:
1) Adhesive wear
2) Abrasive wear
3) Corrosive wear
4) Surface fatigue.
Among those, the type in which the chemical reactions apparently play an important role is corrosive wear. In other types, especially in abrasive wear, mechanical conditions are more influential than the chemical ones; i.e. the wear mode is determined mainly by rather simple parameters such as the degree of penetration and normalized shear strength [2]. In surface fatigue, the stress and number of cycles are dominant parameters [3].
On the other hand, judging from the fact that adhesive (sliding) wear rate is significantly decreased by the oxidation of worn surfaces [4], factors other than mechanical should be taken into account to elucidate wear mechanisms. When the wear of metals is compared between in air and in vacuum, it is apparent that the former is often much higher [5]. These facts indicate that even an ordinary wear between metal and metal in air should inherently be subjected to the chemical reactions with environmental gases which leads to complex results. Nevertheless of the apparent facts, environmental effects, as Tabor pointed out, have not been fully incorporated in the proposed mechanisms of adhesive wear [6].
The reasons for the difficulty in complete understanding of chemical effects on adhesive wear of metals are as follows:
1) One parameter changes with others in a complex manner,
2) There are many implicit parameters, which substantially affect wear,
3) Wear mode associated with sliding conditions changes naturally during sliding.
The example of the first one is that temperature is increased with increasing the sliding velocity [7]. As for the second reason, one of the representative involved parameter is humidity where the wear experiment is carried out [8]. Thirdly change of wear mode is often observed in many tribological pairs [9]. This is called running-in or wear-in process and are typical phenomena associated with adhesive wear [10].
Thus the general questions to be answered concerning the chemical reactions and adhesive wear are as follows:
1) How is wear governed by chemical reactions with environmental molecules?
2) What is the relation between chemical reactions and wear?
3) By what processes do severe and mild wear exhibit quite different phenomena?
The general approach of the present paper is to bridge the gap between wear and chemical reactions. Accordingly, it relates to bridge the gap between macro- and nano-scale phenomena. The key point is the nature of tribofilms, that are formed through chemical interactions of materials with environment as these are micro-scale phenomena. Chemical interactions are initiated by exo-electrons, which are emitted by the wear of materials [11]. Then we propose the feedback cycle; wear ® exo-electron emission ® chemical reaction ®tribofilm ® wear ® exo-electron… Another point of the present theory is to explain chemical reactions due to wear by NIRAM (Negative-Ion-Radical Action Mechanism) approach proposed by Kajdas [12]. The feedback cycle would completely be established by the incorporation of NIRAM approach into the present theory.
To establish the consistent model by eliminating unpredictable factors, one has to construct a specific experimental set-up with particular attention on the only one parameter to be variable while others are kept constant. We introduce here some experiments to extract important parameters for these phenomena. This paper mainly deals with the formation process of adhesive wear particles and the process of severe-mild wear transition. Finally we discuss the relation between wear and chemical reactions.
2. Mechanism of Severe Wear; Role of Contact and Non-contact Zone and Time
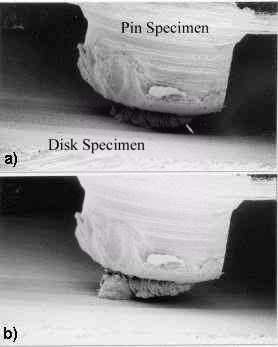
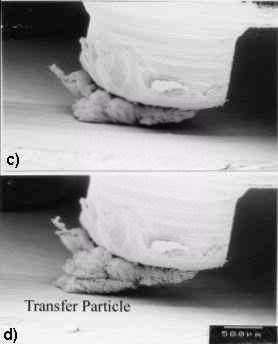
|
Fig. 1: Growth of transfer particle in the rubbing between tin and tin observed by SEM [15]
- Sliding; in ambient air,
- Observation; in vacuum,
- Each photograph (a ¸ d) was taken after every 375mm of the sliding distance,
- Sliding velocity = 2.5 mm/s,
- Load = 2.7N
|
The wear of almost all the materials is affected by the atmospheric oxygen. The effect is significant when self-mated metals are in sliding contact. The friction of metals is decreased by the presence of oxygen on to the surface [13]. This is because the chemisorption of oxygen or oxide prevents metals from adhesion or junction growth; i.e. atmospheric oxygen inhibits metallic interactions. Then it is expected that the chemisorption of oxygen decreases wear as well, because it decreases the adhesion between metals. However, in most metal combinations wear rate is higher in oxygen than in vacuum [14]. This means that the chemical reactions of metal with oxygen relate to the most influential factor on wear. It is observed from the rubbing between tin and tin that the transfer particle grows larger in oxygen atmosphere than in vacuum as shown in Fig.1 [15]. In this case, sliding test was carried out in air. After every 375 mm of sliding distance, the experiment was stopped, the chamber was evacuated and the picture around the interface was taken. This wedge formation against the sliding direction was at first demonstrated by Cocks [16] and Antler [17]. Later Sasada called this phenomenon "mutual transfer and growth process" to explain some typical characteristics in adhesive wear listed below [18]:
1) Mutual transfer on to the mating surfaces,
2) Mixed structure of wear particles from both materials,
3) Wear particle size larger than the real contact area,
4) Mechanism of seizure.
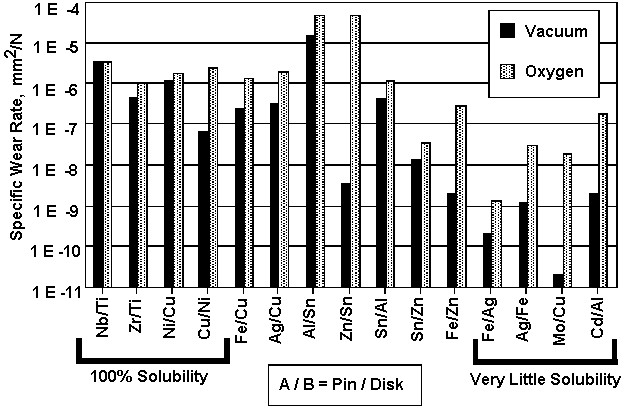
|
Fig. 2: Specific wear rate of each combination in oxygen (1.5x105Pa Oxygen20%+Argon80%) and in vacuum (4x10 -3 Pa) [14]
Load = 9.8N, Sliding Velocity = 33mm/s
|
Fig.2 shows the comparison of the specific wear rate among various combinations in oxygen and in vacuum [14]. As is shown in this graph, in all the sliding couples except of the niobium/tantalum the specific wear rate is higher in oxygen as compared to that in vacuum. The difference is as much as four orders of magnitude in some combinations.
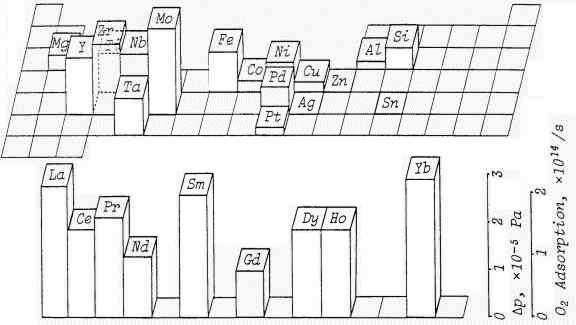
|
Fig. 3: Oxygen adsorption during sliding between self-mated metals [19]
Oxygen Partial Pressure; 1.2x10 -4Pa, Load = 4.2N, Sliding Velocity = 42mm/s
|
During adhesive wear process, atmospheric oxygen adsorbs on the surface. Fig.3 shows the adsorption rate of oxygen onto worn surface of metals when they are in rubbing contact with itself [19]. The order of the rate essentially decreases as shown below:
rare earth metals > transition metals > non-transition metals.
At that point the following question arises. What is the role of consumed oxygen molecules in wear processes?


|
Fig. 4: Wear particles from the rubbing between tin and tin produced in various oxygen pressures [21]
- 1 x 10 -4 Pa O2,
- 3 x 10 -1 Pa O2,
- 2.0 x 10 4 Pa O2,
- 1.5 x 10 5 Pa O2,
- Load = 9.8N,
- Sliding Velocity = 33mm/s
|
Hiratsuka showed that when the ambient oxygen pressure increases, the size of wear particles becomes larger and their hardness increases [20]. Fig.4 shows the wear particles of tin produced in various ambient oxygen pressures. The hardness of transfer particles is higher than that of the bulk material [21] as illustrated in Fig.5.

|
Fig. 5: Cross section of the pin, transfer particle and disk after the rubbing between tin and tin and hardness distribution [21]
- Load = 9.8N,
- Sliding Velocity = 33mm/s
|
He also pointed out that the hardness gradient from surface to inside the bulk is important for the production of wear particles [22]. If the hardened film is thick, the growth will be inhibited. Summarizing the results, atmospheric oxygen helps to grow the transfer particles by the oxygen inclusion hardening at the surface or in sub-surface. Thus, chemical reactions of materials with environmental oxygen strengthen the tribofilm by enhancing the growth of transfer particles.
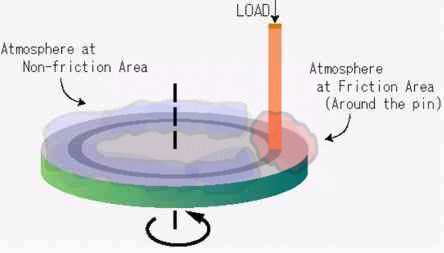
|
Fig. 6: Control of atmospheres at friction area and non-friction area
|
Then, other questions are to be considered. Which region of the wear track does provide the atmosphere affect? Does it relate to the friction (contact) region or non-friction (non-contact) region, as illustrated in Fig.6? What is the role of the atmosphere at each region? The latter question encompasses the following points of interest:
1) Role of the atmosphere at friction and non-friction regions [23],
2) Role of the interval time of the non-friction period.
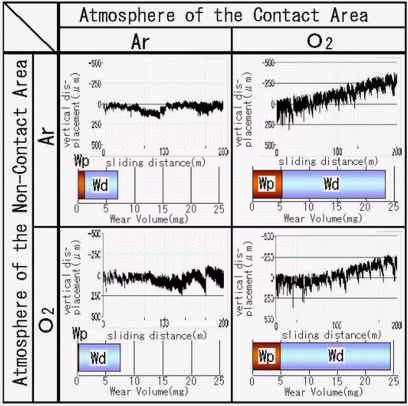
|
Fig. 7: Effect of environment at contact area on wear of Zn and Zn
- WP : wear volume of the pin
- WD : wear volume of the disk
|
When only the contact region is surrounded by inert atmosphere such as argon while the other part is exposed to ambient air, the transfer particles do not grow and almost no wear is obtained in the rubbing between SUJ2 steel and tin [23]. As shown in Fig.7, the vertical displacement of the pin to disk is deactivated by keeping the friction region in inert atmosphere. On the contrary, oxygen atmosphere enhances the growth of transfer particles, resulting in the wear increase. This result shows that the atmosphere at the sliding interface is important for the transfer particle to grow large. On the other hand, the atmosphere at non-friction region does not directly affect the growth.
However, for active metals such as titanium, non-friction region or non-friction time are expected to have some effect on wear. To make clear what is the role of non-friction region and non-friction time in wear of titanium, Hiratsuka carried out a simple experiment. The test procedure for the experiment was as follows:
1) The rotation of disk was stopped after each revolution.
2) It was kept for some fixed time intervals (0, 1, 10, 50, 100 sec.) while pin was paused on the disk.
3) Then the experiment was started again.
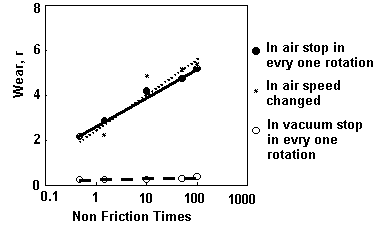 |
Fig. 8: Effect of non-friction time on wear between titanium and titanium in air and in vacuum and effect of sliding velocity on wear in air
- Load = 10N
- Stopping Time in Every Rotation : 0, 1, 10, 50, 100 sec.
- Corresponding Sliding Velocity: 100, 33, 4.8, 0.99, 0.5 mm/s
|
In this intermittent sliding, the sliding velocity was kept constant (100 mm/s). Experiments were carried out in air and in vacuum of 10 -1 Pa. For comparison, continuous sliding tests were also done where velocity was varied like in usual tests. The time for one revolution for each sliding velocity was adjusted to be the same as the intermittent sliding. Total wear is plotted against the interval time (non-friction time) as shown in Fig.8. It is clear that wear is increased to the logarithm of non-friction time. The rate of increase is larger in air than in vacuum. The size of wear particles was smaller when produced in longer non-friction time.
The mechanism is proposed as follows. In non-friction period, the chemical reaction proceeds from surface into sub-surface to modify sub-surface to be brittle. The film grows in a logarithmic function with time. At the moment of sliding, this layer is broken into pieces as small wear particles. The result is explained by assuming that the grow rate of the film is an inverse exponential function with film thickness. Thus, if we assume that
where D is the reaction film thickness,
A and a are constants,
we get that the reaction film thickness is proportional to the logarithm of time D a ln t
and this is in line with experimental results.
From Fig.8 it is also clear that the effect of sliding velocity can be interpreted as the effect of non-friction time; effect of high velocity is the effect of short period of non-friction and vice-versa. The deviation of the plots from the intermittent test should be due to the side effect associated with high velocity, e.g. temperature.
At this point we must note that there are two sets of time/zone that are important from the viewpoint of chemical reactions. These particulars relate to when and where the environmental molecules react with the surface. Two time/zone particulars have different effects on wear:
1) Friction time/zone that provides enhancement of the growth of transfer particles,
2) Non-friction time/zone that effects a decrease in the size of wear particles and increase in the number of them.
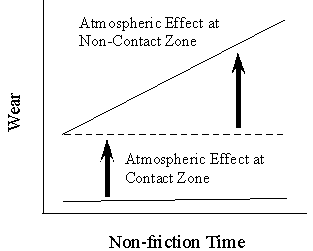
| |
Fig. 9: Schematic of the role of atmosphere at contact and non-contact zones
|
Effect of each time/zone on wear is different due to the different inorganic tribofilm formation. For example, at the moment of sliding when oxygen adsorbs, shearing force rolls up the adsorbed layer to make mixed structure at sheared zone. The shear strength promotes the growth of transfer particles to give rise to relatively larger wear particles. On the other hand, during the interval period, hydrogen/oxygen penetrates into the bulk to make a stable compounds which significantly reduce ductility of metals and embrittle the surface tribofilm. It enhances the brittle fracture when the mechanical action is applied at the moment of friction. We understand now that the surrounding atmosphere should have different role at contact area and non-contact area. However in both cases wear is increased in a way of the inorganic tribofilm formation. This is illustrated in Fig.9.
The interval time also provides an interesting effect on friction. The coefficient of friction of diamond like carbon increases as the interval time is increased logarithmically [24]. In this case the relationship between coefficient of friction and the amount of gas chemisorption on the surface correlated with each other.
3. Role of Chemical Reactions in Severe-mild Wear Transition. Importance of Tribofilm
When two metals are in sliding contact, initial severe wear is followed by mild wear. Severe and mild wear are quite different in the nature of wear particles; severe wear particles are metallic whereas mild wear particles are composed from oxides [25]. In mild wear regime, worn surfaces are covered by the oxide film and they prevent the tribosystem from seizure which leads to the cease of the whole system. The wear rate of mild wear is usually some orders of magnitude less than that of severe wear [26], although in some cases two modes exhibit nearly the same wear rate [27]. Therefore from the practical point of view, mild wear regime is favorable for longer operation time and for anti-seizure. To accelerate the severe-mild wear transition and maintain the mild wear regime, it is necessary to understand the mechanism of that transition.
The principal cause for mild wear is the formation of protective anti-wear film on the surface. However the sequence or mechanism of the film formation has not been clear. Taking an example from the wear between copper and iron in a dry condition at ambient temperature, the importance of the attachment of oxide on the surface is demonstrated as the key mechanism of severe-mild wear transition by the following simple experiments [28].
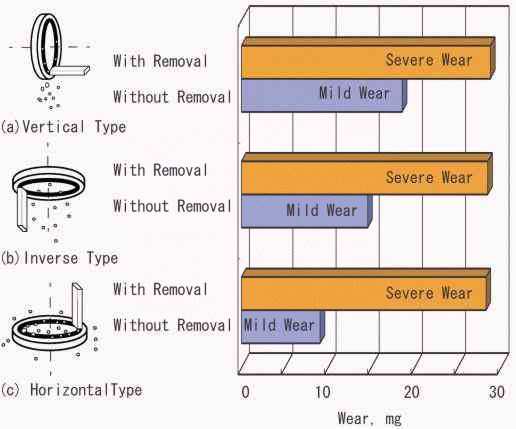 |
Fig. 10: Effect of removal of wear particles and direction of friction plane on wear between copper pin and iron disk
- Load = 5.9N,
- Sliding Velocity = 50mm/s,
- Sliding Distance = 200m
|
Using pin-on-disk test rig (copper pin vs. iron disk), the wear particles produced on the disk surface were continuously removed by air cleaner. Then severe wear continued and did not transform into mild wear state [28]. When the wear particles naturally remain on the surface by setting the disk specimen horizontally with pin specimen standing vertically, the wear was decreased more than in any other configuration of friction components, as shown in Fig.10. This result means that the attachment of oxide is important, i.e. the cohesion of oxide on the surface protects the surface from wear.
Mild wear particles are found as sliding proceeds even in a severe wear mode [28]. This means that mild wear particles are the precursors for the mild wear mode. To summarize, individual steps of the wear mode transition process from severe to mild wear can be demonstrated as follows:
1) Production of severe wear particles,
2) Production of mild wear particles (oxide formation),
3) Attachment of mild wear particles (oxide growth),
4) Prevalence of mild wear region over the surface (Oxide prevalence)
5) Transition from severe to mild wear regime.
Next we will discuss the key parameter, which determines the wear transition from severe to mild wear.
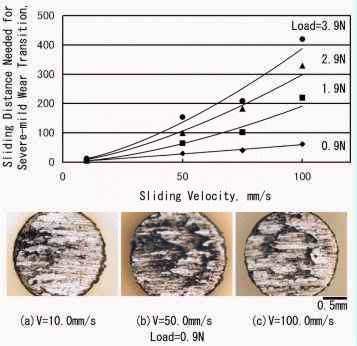 |
Fig. 11: Effect of sliding velocity and load on severe-mild wear transition distance in the rubbing between copper pin and iron disk
|
As shown in Fig.11, by increasing the sliding velocity at constant load, the sliding distance needed for severe-mild wear transition is increased in proportion to the sliding velocity. This means that the most important variable for transition is the total time of sliding, because the time for severe-mild wear transition is the same for different sliding velocities. It was also found that the sliding distance needed for severe-mild wear transition is in proportion to the applied load. From the simple relation that real area of contact is also in proportion to the load, it is deduced that a certain amount of accumulation of mild wear particles determines the severe-mild wear transition. Thus the severe-mild wear transition is modeled as follows:
1) Mild wear particles are produced at a constant rate per unit area per unit time in severe wear mode.
2) A fixed ratio of them are attached onto the sliding surface.
3) When the thickness of the accumulation exceeds a critical level, which prevails over larger area than the real area of contact, the wear mode transforms into mild.
As shown above, the key point of this phenomenon is the attachment of mild wear particles on the worn surface. In some experimental conditions, mild wear state is not so stable because the two wear modes appear alternatively. The severe and mild wear are sensitive to the sliding conditions. For example, by the slight change of load and velocity, the wear mode changes substantially, sometimes as much as four order of magnitude increase in wear [29].
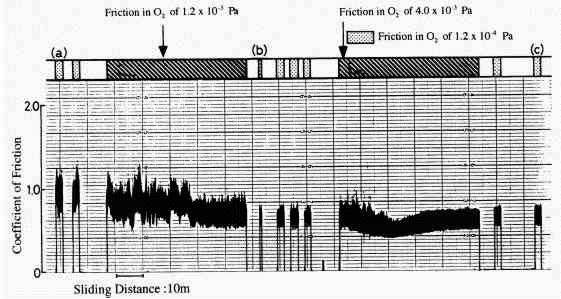
|
Fig. 12: Pressure changes associated with friction in severe and mild wear modes before and after the friction in oxygen [30]
- Chemisorption of O2 (Severe Wear),
R1 = 6,7 x 10 13 S -1
- Chemisorption of O2 (Severe Wear),
R2 = 1,1 x 10 14 S -1
- Chemisorption of O2 (Mild Wear),
R3 = 1,9 x 10 14 S -1
- Load = 2.6N,
- Sliding Velocity = 42mm/s,
- Ambient Temperature = 230C
|
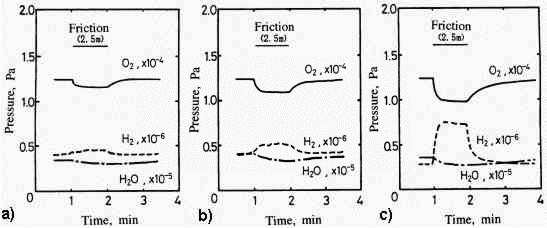
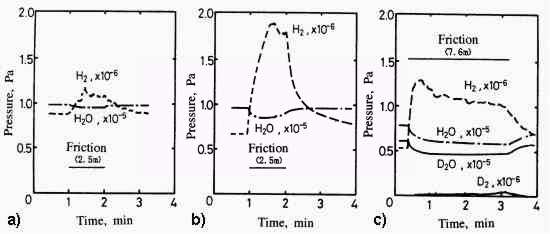
The adsorption behavior of gases onto metals implies the formation mechanism of mild wear mode [30]. As shown in Fig.12, in severe wear, oxygen mainly adsorbs onto the iron surface. On the other hand, after the mild wear mode is established, oxygen and water adsorb more and hydrogen is evolved much more. This hydrogen originates apparently from water. However it is not the direct conversion of adsorbed water but the absorbed water gradually decomposed into hydrogen and then it evolved. It is proved from the experiment using heavy water [30] as shown in Fig.13.

|
Fig. 13: Pressure changes associated with friction in severe and mild wear modes before and after the friction in oxygen with special attention on the effect of D2O [30]
- Chemisorption of H2O (Severe Wear),
R1 = 1,8 x 10 13 S -1
- Chemisorption of H2O (Mild Wear),
R2 = 7,9 x 10 13 S -1
- Chemisorption of D2O (Mild Wear)
- Load = 2.6N,
- Sliding Velocity = 42mm/s,
- Ambient Temperature = 230C
|
Desorption of deuterium is much less than hydrogen although the adsorption of water and heavy water are the same. The reaction is initiated by exo-electron emission associated with water and the scheme would be formulated as follows:
H2O + e ® HO - (hydroxide anion formation) + H· (absorption)
H· + H· ® H2 (desorption)
Judging from the water consumption in mild wear mode, it is hypothesized that the key point of mild wear formation might be the termination of the oxygen chemical bond by hydrogen, which leads to the hydroxide. This hypothesis is supported by the fact that in the case of aluminum the hydroxide formation on aluminum greatly reduces wear in humid air conditions [31].
4. Chemical reactions enhanced by wear. Tribocatalysis
As discussed above, chemical reactions affect wear through the formation of tribofilm. On the other hand some questions arise.
1) Are there opposite phenomena?
2) Does wear affect chemical reactions?
3) What is the mechanism of it?
We will explain those by simple chemical reactions between gas and gas. The reason for choosing simple reactions is as follows. If the reaction product covers the friction surface to make a film, it changes the wear mode depending on the mechanical property of the film whether it is protective or destructive. Consequently it changes the chemical reactions. This feedback loop makes us difficult to elaborate the scheme of chemical reactions enhanced by wear. So we picked up simple cases where the reaction products do not stay on the surface but evaporate from the surface.
|
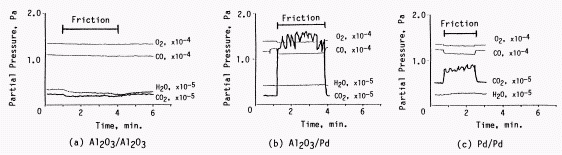
Fig. 14: Pressure changes associated with the rubbings of Al2O3/Al2O3, Al2O3/palladium and palladium/palladium [33]
Load = 2.6N, Sliding Velocity = 42mm/s
|
The water formation from hydrogen and oxygen is extensively discussed in this book [32]. Here we introduce another example; the formation of carbon dioxide from carbon monoxide and oxygen being enhanced by the friction between palladium and itself or aluminium oxide, which is shown in Fig.14 [33]. In this case the catalytic activity is enhanced when the catalyst is in sliding contact. The load and sliding velocity is so small that we can neglect the effect of frictional heat. The reason of the activation must be due to the other mechanisms besides heat.
| |
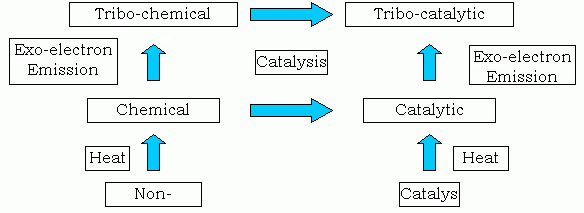
Fig. 15: Correlation of chemical, tribo-chemical, catalytic and tribo-catalytic reactions
|
We propose in Fig.15 the correlation of four reactions of tribochemical, thermochemical, catalytic and tribocatalytic. Any substance is classified into two groups, catalyst or non-catalyst. The catalytic activity emerges when it is heated up to some temperature. On the other hand, without heat, when the catalyst is in sliding contact even in a mild condition, the catalytic activity exhibits. That is to say, heat and friction have the same effect. The interpretation of the phenomena is as follows. Catalytic reaction is activated by the energy injection to the system such as heat etc. Tribocatalytic reaction is activated by low energy electrons emitted from the worn surface. However one might unify those different reaction schemes from the point of view of the low energy electron emission [34]. In normal catalyst, when it is heated up, thermal electrons of low energy are emitted from the surface. From the energy point of view, these electrons are similar to exoelectrons emitted from the rubbing surface during wear processes. A kind of similar relationship would be applied to the chemical (thermochemical) and tribochemical reactions. The reaction enhanced by wear is due to the increased emission of electrons from the worn surfaces. If we call wear itself is a catalysis, any substance becomes a catalyst when it is in sliding contact.
5. Correlation of Wear, Chemical Reactions and Tribofilm.
Open and Closed Cycle of Tribological Interactions
| |
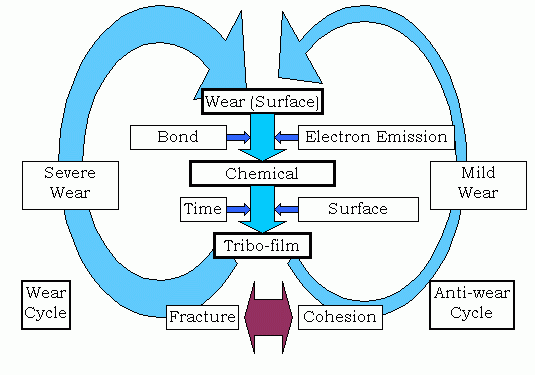
Fig. 16: Reaction schemes of two different cycles of severe and mild wear
|
From the discussion above, the correlation of wear, chemical reaction and tribofilm is summarized in Fig.16. By the bond breakage low energy electrons are emitted [11]. It triggers the chemical reactions. For the subsequent surface modification processes, time is needed to form the tribofilm. Then the mechanical properties of tribofilm determine the direction of the system toward wear or anti-wear. When the tribofilm is brittle in nature and is destructive by the mechanical stimulation, it breaks off due to mechanical action. The particles will not return to the original surface resulting in production of wear particles. Then new surface is generated and the initial reactions are repeated. In this case, wear is in proportion to the sliding distance. This is called "severe wear mode" or simply "wear mode". On the contrary, if tribofilm is non-destructive and cohesive, it protects the subsurface, which enhances the anti-wear property of the system. This is called "mild wear mode" or "anti-wear mode". The different cycles induce completely different wear modes. The severe-mild wear transition is interpreted as the shift of the cycle from wear mode to anti-wear mode in the diagram of Fig.16.
The reason why the tribological phenomena are so complex is that a series of reactions construct feedback cycles, which result in the change of surface properties and wear rate. If tribofilm formation stabilizes the system to inhibit wear, it also inhibits the formation of tribofilm itself. Then the system might come back to promote wear. This kind of alternation between wear and anti-wear modes is the essential point of wear phenomena.
The reason of this instability is due to the scale difference between wear and chemical reactions. Wear is the phenomenon in visible level, whereas chemical reactions are in atomic scale. There is a scale difference of some orders of magnitude. The bridge of the two scales is tribofilm, which is in microscopic level. Atomic scale phenomena are transformed into macroscopic ones through the incubation of tribofilm during non-friction period. The closed cycle of "wear ® chemical reaction ® tribofilm ® wear ® chemical reaction…" is actually the open system. Chemical reactions of sliding materials with environmental molecules promote this cycle yielding wear particles by involving reaction materials such as sliding materials and environment.
Wear and chemical reactions appear concurrently at the moment of sliding. After the contact, surface is an incubation period to prepare the brittle or ductile film, which would lead to wear or anti-wear, respectively. When the film is in contact again, it chooses the way to break off or support load and shear. Tribological interactions are the surface phenomena where macroscopic, microscopic, and atomic level phenomena coexist, and are enhanced by time.
6. Conclusions
From the experimental results and discussions regarding the adhesive wear and chemical reactions mentioned above, the following conclusions have been drawn.
- Chemical reactions of sliding materials with environmental molecules influence the occurrence of tribofilm formation.
- Tribofilm is not only formed at the instant of contact but also incubated during the interval time between, after and before the contact.
- The mechanical properties of tribofilm determine the occurrence of severe or mild wear.
- Breakage of cohesive bonds associated with wear induces exo-electron emission and enhances chemical reactions.
- The cyclic process which consists of; wear ® exo-electron emission ® chemical reaction ® tribofilm formation ® wear ®…, and time for the process are the key concepts for better understanding of wear phenomena.
| |
References
- [1]
- J.T.BURWELL,Jr.: Survey of Possible Wear Mechanisms, WEAR, 1 (1957) 119-141
- [2]
- K.HOKKIRIGAWA, K.KATO: An Experimental and Theoretical Investigation of Ploughing, Cutting and Wedge Formation during Abrasive Wear, Tribology International, 21, 1 (1988) 51-57
- [3]
- J.M.CHALLEN, P.L.B OXLEY, B.S. HOCKENHULL: Prediction of Archard's Wear Coefficient for Metallic Sliding Friction Assuming a Low Cycle Fatigue Wear Mechanism, Wear, 111 (1986) 275-288
- [4]
- J.K.LANCASTER: The Formation of Surface Films at the Transition Between Mild and Severe Metallic Wear, Proc. Roy. Soc. London A, 273 (1963) 466-483
- [5]
- H.MISHINA: Atmospheric Characteristics in Friction and Wear of Metals, Wear, (1992) 99-110
- [6]
- D.TABOR: Status and Direction of Tribology as a Science in the 80's Understanding and Prediction, Proc. International Conf. Tribology in the 80's, NASA Lewis Research Center, Cleveland, Ohio, NASA. (1983) 1-17
- [7]
- S.C.LIM, M.F.ASHBY: Wear-mechanism Maps, Acta metal, 35, 1 (1987) 1-24
- [8]
- K.FUKUDA, S.NOROSE, T.SASADA: Effect of Humidity on Sliding Wear in Fe and Cu Rubbing System, Proc. 28th Japan Congress Mater. Res., (1985) 289-292
- [9]
- H.SO, D.S.YU, C.Y.CHUANG: Formation and Wear Mechanism of Tribo-Oxides and the Regime of Oxidational Wear of Steel, WEAR, 253 (2002) 1004-1015
- [10]
- P.J.BLAU: Friction and Wear Transitions of Materials, Break-in, Run-in, Wear-in, Materials Science and Process Technology Series, Noyes Pub. (1989)
- [11]
- G.J.MOLINA, M.J.FUREY, A.L.RITTER, C.KAJDAS: Triboemission from Alumina, Single Crystal Sapphire, and Aluminum, Wear, 249 (2001) 214-219
- [12]
- C.KAJDAS: Importance of Anionic Reactive Intermediate for Lubricant Component Reactions with Friction Surfaces, Lubrication Science, 6-3 (1994) 204-228
- [13]
- D.H.BUCKLEY: Influence of Chemisorbed Films of Various Gases on Adhesion and Friction of Tungsten, Journal of Applied Physics, 39, 9 (1968) 4224-4233
- [14]
- K.HIRATSUKA, A.SUGAHARA: The Role of Atmospheric Oxygen in Friction and Wear between Dissimilar Pure Metals, Japanese Journal of Tribology, 34, 11 (1989) 1256-1265
- [15]
- K.HIRATSUKA: Scanning Electron Microscope, Tribology Handbook, Japanese Society of Tribologists Ed, (2001) 394-397
- [16]
- M.COCKS: Interaction of Sliding Metal Surface, Journal of Applied Physics, 33, 7 (1962) 2152-2161
- [17]
- M.ANTLER: Processes of Metal Transfer and Wear, WEAR, 7 (1964) 181-203
- [18]
- T.SASADA: Wear Research in Japan: Trends and Future Directions, Wear, 100 (1984) 561-577
- [19]
- T.SASADA, K.HIRATSUKA, H.SAITO: Adsorption of Surrounding Gas Molecules on Pure Metal Surfaces during Wear Processes, Wear, 135 (1990) 251-264
- [20]
- K.HIRATSUKA. Environmental Effect on the Formation Process of Adhesive Wear Particles, Tribology International, 28,5 (1995) 279-286
- [21]
- K.HIRATSUKA?A.SUGAHARA: The Effect of Oxygen on the Growth of Transfer Particles During the Rubbing of Sn / Sn and Zn / Zn, Transactions of the Japan Society of Mechanical Engineers (C), 66?641, (2000) 291-299
- [22]
- K.HIRATSUKA, M. GOTO: The Role of Changes in Hardness of Subsurfaces, Transfer Particles and Wear Particles in Initial-Steady Wear Transition, Wear, 238 (2000) 70-77
- [23]
- K.HIRATSUKA, M.WAKASA: Role of Environment around the Contact Zone in Wear of Metals, Proc. International Tribology Conference Nagasaki, (2001) 367-369
- [24]
- J.A.HEIMBERG, K.J.WAHL, I.L.SINGER, A.ERDEMIR: Superlow Friction Behavior of Diamond-like Carbon Coatings: Time and Speed Effects, Applied Physics Letters, 78, 17 (2001) 2449-2451
- [25]
- T.SASADA: Fundamental Analysis of the "Adhesive Wear " of Metals -Severe and Mild wear-, Proc. JSLE International Tribology Conference, (1985) 329-334
- [26]
- T.SASADA, H.OHMURA, S.NOROSE: The Wear and Mutual Transfer in Cu / Fe Rubbing, Proc. 15th Japan Congress on Materials Research, 1 (1972) 1-6
- [27]
- H-S HUNG: The Role of Atmospheres and lubricants in the oxidational wear of metals, Tribology International, 35 (2002) 725-729
- [28]
- K.MURAMOTO, B.PRAKASH, K.HIRATSUKA: Influence of Wear Particles Removal on Friction and Wear of Metals, Proceedings of the International Tribology Conference Nagasaki, (2001) 363-366
- [29]
- T.SASADA, S.NOROSE: The Dependence of Wear Rate on Sliding Velocity and Sliding Distance for Dry Cu / Fe and Ni / Fe, Proc. International Conference Wear of Materials, (1985) 302-306
- [30]
- K.HIRATSUKA, H.SAITO, T.SASADA: Gas Chemisorption onto Metal Surface during Adhesive Wear (Severe-Mild Wear Transition and Chemisorption Activity in the Rubbing between Iron and Itself) , Transactions of the Japan Society of Mechanical Engineers (C), 66, 648 (2000) 2818-2824
- [31]
- M.G.GEE: The Formation of Aluminium Hydroxide in the Sliding Wear of Alumina, Wear, 153 (1992) 201-227
- [32]
- C.KAJDAS, K.HIRATSUKA, M.HASHIMOTO: Mechanism of Water Synthesis under Pt/Pt Tribological System in Vacuum, in this book.
- [33]
- K.HIRATSUKA, M.KUZUYA, T.SASADA: Friction Catalysis in the Synthesis of CO2 during Adhesive Wear, Proc. 34th Japan Congress on Materials Research (1991) 119-123.
- [34]
- C.KAJDAS: Tribochemistry. In: Tribology 2001 - Scientific Achievements, Industrial Applications, Future Challenges, 2nd World Tribology Congress, Eds. F. Franek. W.J. Bartz. A. Pauschitz. The Austrian Tribology Society, Vienna (2001), ISBN 3-901657-07-X, 39-46.
| |



















